UK scientists have used synchrotron X-ray techniques to help develop a catalyst that converts biomass into fuel efficiently, potentially leading to a more sustainable source of petrochemicals for use in industry.
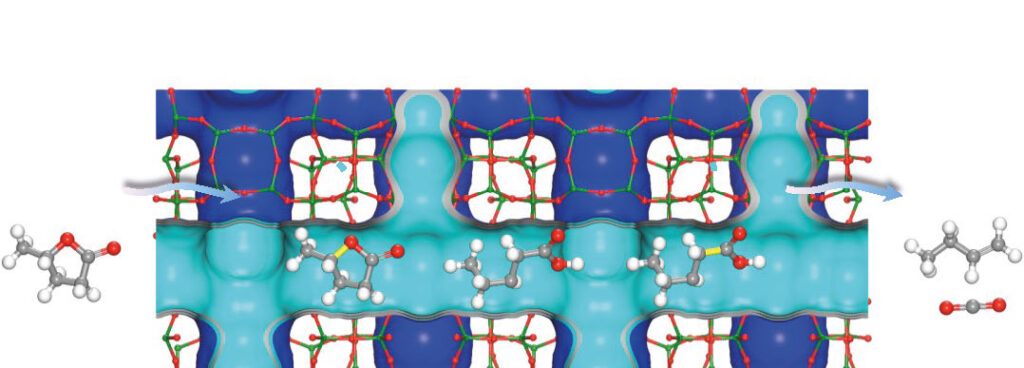
Converting biomass into light olefins – the group of petrochemicals that includes ethene, propene and butene – requires a catalyst and a lot of energy to convert the waste from organic matter such as wood or grass. Researchers led by the University of Manchester designed a highly efficient zeolite catalyst that uses less energy to produce the olefins from renewable biomass sources.
The team tested the zeolite – a porous material often used in catalysis – at the UK’s Diamond Light Source, the Science and Technology Facilities Council’s ISIS Neutron and Muon Source and the US Department of Energy’s Oak Ridge National Laboratory to determine its atomic structure.
The zeolite, called NbAlS-1, has an impressive yield of more than 99% but requires significantly less energy compared to its predecessors. It can be used to convert leftover agricultural waste-derived raw materials into butene, an energy-rich gas used by the chemical and petroleum industries to make plastics, polymers and liquid fuels traditionally derived from crude oil with its associated environmental impacts. The team’s research is published in the journal Nature Materials.
The scientists designed the zeolite to require less energy to break the strong bonds formed from elements such as carbon, oxygen, and hydrogen by replacing its silicon atoms with niobium and aluminum. The substitution creates a chemically unbalanced state that promotes bond separation and radically reduces the need for high degrees of heat treatments.
Synchrotron X-ray absorption/diffraction measurements at the UK’s Diamond Light Source were used to determine the catalyst’s atomic structure and interaction between biomass derived γ-valerolactone and the zeolite. XAFS measurements were performed during a BAG beamtime (UK Catalysis Hub SP15151) at Diamond Light Source. XAFS has been demonstrated as a powerful technique to study the coordination environment of Nb centers in zeolites. It confirms the location of Nb(V) to be solely within the framework. The change of local structure of Nb sites on adsorption of γ-valerolactone was revealed by XAFS. These XAFS studies give a strong evidence that Nb sites play a key role on the adsorption and activation of γ-valerolactone and provide further insight into the reaction mechanism.
“There’s a lot of trial and error associated with designing such a high-performance catalyst such as the one we’ve developed,” said corresponding author Sihai Yang at the University of Manchester. “The more we understand how catalysts work, the more we can guide the design process of next-generation materials.”
Reference:
Lin, L., Sheveleva, A.M., da Silva, I. et al. “Quantitative production of butenes from biomass-derived γ-valerolactone catalysed by hetero-atomic MFI zeolite.”, Nature Materials (2020), https://doi.org/10.1038/s41563-019-0562-6




