BAG at Diamond Light Source
Block Allocation Group (BAG) Programme Mode Application to Diamond Light Source:
The BAG explained
The UK Catalysis Hub BAG aims to provide members of the Hub network and all groups doing catalytic science in the UK with frequent and flexible access to B18, sending out two calls for proposals per cycle. One advantage of sending out two calls for proposals per cycle, is that our users can obtain beamtime on a rapid turnaround, which has been especially useful when additional measurements are required on a short timescale to finish pieces of work for publication.
The dates of calls for proposals are set by Diamond light source and will be sent to the Mailing list, to sign up for the mailing list here.
We are committed to increasing the user base of XAFS in the catalyst community, and so in part the BAG works as a training scheme. The team at Harwell guides new users through the proposal and experimental stages, showing them the potential of XAFS for their projects and training them on the analysis of their data. We ask all PI to be named on their proposal and also to indicate which member of their group will be responsible for the beamtime measurements, and who will learn how to analyse the data
Submitting a proposal
BAG Call Open
Our BAG Call for B18 at Diamond Light Source is open. This is the call for proposals for the next beamtime allocation at B18 as part of the UK catalysis Hub BAG access. This is the first call for proposals for the next beamtime allocation at B18. The next session is scheduled for 26th to 29th September 2025. For more information visit the call page.
Ex Situ
Ex situ samples are generally made from powder, pressed into pellet form, however liquids, films and powders can also be measured. To submit a proposal for in situ measurement, please fill in the form at https://form.jotform.com/250512886562360 which can be accessed when a call is open.
Please feel free to discuss any proposed ex-situ measurement with the BAG management team prior to submission.
In Situ
All in situ experiments must be discussed with the BAG management team beforehand.
Please contact the BAG officer: Dr Martin Wilding (wildingM2@cardiff.ac.uk) to set up a meeting with the EXAFS experts in the Hub. After this initial discussion we will send you an in situ application form.
Please note: proposals must involve UK academics in the catalysis community. Proposals with international collaborators must be submitted by the UK collaborator(s). The proposals must also be catalysis focussed or on the development of cells/ methodologies for catalysis experiments.

image credit: UK Catalysis Hub
Process
Submitted proposals are assessed by a panel, comprising representation from Hub scientists, and representatives from two other institutions (changed on a rolling basis). The latter positions are filled by researchers in catalysis, one of whom usually has XAFS experience. The BAG allocation prioritises access based on:
- scientific excellence,
- feasibility,
- attracting new users and new areas of catalytic science, and
- maximising efficiency.
Both in situ /operando studies and standard ex situ (rapid access and proof of concept) measurements are facilitated, with generally an even mix of the two proposal types being awarded time. Due to our aim of attracting new and inexperienced users to XAFS, proposals are not often accepted ‘as is’ but redesigned to make the best use of time and provide the user with the measurements they need. In this way, we plan the BAG shifts to facilitate the greatest number of projects efficiently. No single user is awarded more than 3 shifts in any one call and we limit experienced users of XAFS to short bursts of time: in this way the BAG should not serve as an ‘easy’ access to B18 for those who should apply through the Direct Access Route.
It is important to note that that for heavy users of synchrotron facilities this method of access will only play a small contribution to the amount of time they require. Their role within the BAG proposal is to provide the interface between the inexperienced members and Diamond, enabling a larger cross section of the UK Catalysis Hub network to benefit from the techniques available. These users may receive small amounts of flexible access, where required, to assist in finishing research projects and hasten subsequent publications.
I have been awarded time in the BAG, what now?
Before the experiment
Session investigators
Once you are notified that your proposal has been awarded time on the BAG, please make sure that you let the BAG coordinator know who on your team will be attending the experiment. All those planning to come to Diamond must be registered in UAS (https://uas.diamond.ac.uk/uas/) and must have watched the health and safety video and completed the test (https://www.diamond.ac.uk/Users/Experiment-at-Diamond/Before-you-Arrive/Safety-Video-and-Test.html).
Experimental Risk Assessment
The Principal Investigator or Alternate Contacts need to submit the ERA (Experimental Risk Assessment) in UAS. All the samples and any equipment that will be brought to Diamond must be specified in the ERA. If it is necessary to bring your own sample environments please discuss this in advance with the BAG coordinator. The ERA template can be found using this document – BAG Risk Assessment.
Travel, subsistence and accommodation
If your scheduled experiment requires overnight accommodation on-site, the BAG coordinator will organise that for you and will let you know the details. However, you will have to organise your own travel arrangements. Please note that Diamond will only cover the subsistence for the Core Team, so you will have to pay for your meals.
Please note that only one person will be funded per ex situ proposal and two per in situ proposal.
To find details about how to travel to Diamond, please visit: https://www.diamond.ac.uk/Users/Experiment-at-Diamond/Before-you-Arrive.html
Sample Shipment
Sometimes it is simpler to ship your samples to us. Samples being shipped should be address to:
BAG coordinator
UK Catalysis Hub, Research Complex at Harwell
Rutherford Appleton Laboratory,
Harwell,
Oxfordshire, OX11 0FA
During the experiment
When you arrive at Diamond you will be given an access card. This is usually at the main security gate to site. The card will be pre-loaded with the appropriate access for your session. If there are difficulties with your card please contact the user office (ext. 8571). Please wear your access card at all times and return the card, along with the lanyard at the end of your session.
Once on the beamline, please follow the instructions given by the BAG coordinator and or local contacts in charge of the experiment. The way the BAG works, it is difficult to follow the scheduled timetable. Please be patient if your experiment is delayed from the initial estimated time.
After your experiment
Experimental Report
All proposals require a report once the session scheduled in that proposal are complete.
In all cases this report should include any changes in methodology that were required and highlight key results as well as indicate the status of the project(s) in light of these results. Experiment Reports are made available to the Peer Review Panel when they are considering further proposals from the same Principal Investigator and failure to submit a report can be considered detrimental for future access.
You can find the report template using this document – BAG Report Template
Publications
Users publishing work containing synchrotron data from Diamond Light Source should ensure they acknowledge Diamond in all publications (including conference proceedings) arising from work carried out wholly or partially at Diamond. The following acknowledgement statement must be included in all published reports:
“The authors wish to acknowledge the Diamond Light Source for provision of beamtime (proposal number).”
Please also inform the BAG coordinator of all Publications arising from the BAG.
Track record
Through SP8071, SP10306, SP15151 and SP19850, 87 days (excluding reassigned days) have been received. Since the first beamtime allocation period, 67 articles have been published, which is an average of <1½ days beamtime per paper; the growth is shown in Fig. 1.
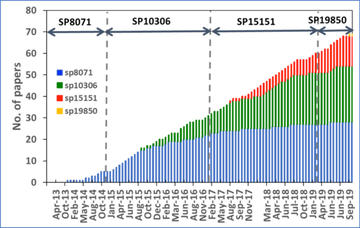
Publications
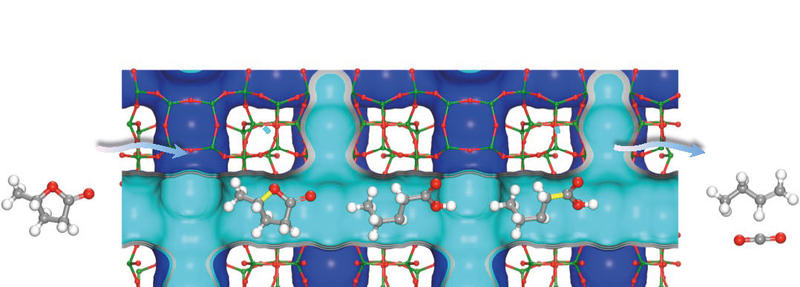
BAG Impact Case Study: Quantitative production of butenes from biomass-derived γ-valerolactone catalysed by hetero-atomic MFI zeolite
UK scientists have used synchrotron X-ray techniques to help develop a catalyst that converts biomass into fuel efficiently, potentially leading to a more sustainable source of petrochemicals for use in industry. Converting biomass into light olefins – the group of petrochemicals that includes ethene, propene and butene – requires a catalyst and a lot of energy to convert the waste from organic matter such as wood or grass. Researchers led by the University of Manchester designed a highly efficient zeolite catalyst that uses less energy to produce the olefins from renewable biomass sources. Read more
-
- Donald R. Inns, Megan Carr, Mounib Bahri, Ajay Tomer, Troy D. Manning, Nigel Browning, Simon A. Kondrat, John B. Claridge, Alexandros P. Katsoulidis and Matthew J. Rosseinsky, Elucidating the effect of nanocube support morphology on the hydrogenolysis of polypropylene over Ni/CeO2 catalysts, J. Mater. Chem. A, 2025, Advance Article, DOI: https://doi.org/10.1039/D4TA08111K
- Longxiang Liu, Liqun Kang, Jianrui Feng, David G. Hopkinson, Christopher S. Allen, Yeshu Tan, Hao Gu, Iuliia Mikulska, Veronica Celorrio, Diego Gianolio, Tianlei Wang, Liquan Zhang, Kaiqi Li, Jichao Zhang, Jiexin Zhu, Georg Held, Pilar Ferrer, David Grinter, June Callison, Martin Wilding, Sining Chen, Ivan Parkin, Guanjie He, Atomically dispersed asymmetric cobalt electrocatalyst for efficient hydrogen peroxide production in neutral media, Nat Commun 15, 4079 (2024). DOI: https://doi.org/10.1038/s41467-024-48209-0
- Raúl Rojas-Luna, Francisco J. Romero-Salguero, Dolores Esquivel, Souvik Roy, Manipulating the coordination structure of molecular cobalt sites in periodic mesoporous organosilica for CO2 photoreduction, ACS Appl. Energy Mater. 2024, 7, 14, 5924–5936, DOI: 10.1021/acsaem.4c01161
- Danial Farooq, Matthew E. Potter, Sebastian Stockenhuber, Jay Pritchard, Antonios Vamvakeros, Stephen W. T. Price, Jakub Drnec, Ben Ruchte, James Paterson, Mark Peacock, Andrew Beale, Chemical imaging of carbide formation and its effect on alcohol selectivity in Fischer Tropsch synthesis on Mn-doped Co/TiO2 pellets, ACS Catal. 2024, 14, 16, 12269–12281, DOI: 10.1021/acscatal.4c03195
- Khaled Mohammed, Reza Vakili, Donato Decarolis, Shaojun Xu, Luke L. Keenan, Apostolos Kordatos, Nikolay Zhelev, Chris K. Skylaris, Marina Carravetta, Emma K. Gibson, Haresh Manyar, Alexandre Goguet, Peter P. Wells, Structural selectivity of supported Pd nanoparticles: selective ethanol ammoxidation to acetonitrile, EES. Catal., 2024,2, 987-996, DOI: 10.1039/D4EY00044G
- Thomas Doughty, Andrea Zingl, Maximilian Wünschek, Christian M. Pichler, Matthew B. Watkins, Souvik Roy, “Structural reconstruction of a cobalt- and ferrocene-based metal-organic framework during the electrochemical oxygen evolution reaction”, CS Applied Materials & Interfaces 2024 16 (31), 40814-40824, DOI: 10.1021/acsami.4c03262
- Ruiz Esquius, Jonathan, Morgan, David J., Algara Siller, Gerardo, Gianolio, Diego, Aramini, Matteo, Lahn, Leopold, Kasian, Olga, Kondrat, Simon A., Schlögl, Robert, Hutchings, Graham J., Arrigo, Rosa, Freakley, Simon J., “Lithium-Directed Transformation of Amorphous Iridium (Oxy)hydroxides To Produce Active Water Oxidation Catalysts”, J. Am. Chem. Soc. 2023, 145, 11, 6398–6409, https://doi.org/10.1021/jacs.2c13567
- Run Zou, Sarayute Chansai, Shaojun Xu, Bing An, Shima Zainal, Yangtao Zhou, Ruojia Xin, Pan Gao, Guangjin Hou, Carmine D’agostino, Stuart M. Holmes, Christopher Hardacre, Yilai Jiao, Xiaolei Fan, “Pt nanoparticles on beta zeolites for catalytic toluene oxidation: effect of the hydroxyl groups of beta zeolite” Chemcatchem (2023), DOI: 10.1002/cctc.202300811
- Priyanka Verma, Kohsuke Mori, Yasutaka Kuwahara, Maela Manzoli, Sara Morandi, Choji Fukuhara, Robert Raja, Hiromi Yamashita, “Amine functionalization within hierarchically‐porous zeotype framework for plasmonic catalysis over PdAu nanoparticles”, Chemcatchem (2023), DOI: 10.1002/cctc.202201182
- Jichao Zhang, Xuedan Song, Liqun Kang, Jiexin Zhu, Longxiang Liu, Qing Zhang, Dan J. I. Brett, Paul R. Shearing, Liqiang Mai, Ivan P. Parkin, Guanjie He “Stabilizing efficient structures of superwetting electrocatalysts for enhanced urea oxidation reactions”, Chem Catalysis (2023), 121, DOI: 10.1016/j.checat.2022.09.023
- Yujie Ma, Xue Han, Shaojun Xu, Zhe Li, Wanpeng Lu, Bing An, Daniel Lee, Sarayute Chansai, Alena M. Sheveleva, Zi Wang, Yinlin Chen, Jiangnan Li, Weiyao Li, Rongsheng Cai, Ivan Da Silva, Yongqiang Cheng, Luke L. Daemen, Floriana Tuna, Eric J. L. Mcinnes, Lewis Hughes, Pascal Manuel, Anibal J. Ramirez-Cuesta, Sarah J. Haigh, Christopher Hardacre, Martin Schroeder, Sihai Yang, “Direct conversion of methane to ethylene and acetylene over an iron-based metal–organic framework”, Journal Of The American Chemical Society (2023), DOI: 10.1021/jacs.3c03935
- Jose Pinto, Andreas Weilhard, Luke T. Norman, Rhys W. Lodge, David M. Rogers, Aitor Gual, Israel Cano, Andrei N. Khlobystov, Peter Licence, Jesum Alves Fernandes, “Unravelling synergistic effects in bi-metallic catalysts: deceleration of palladium-gold nanoparticles coursing in the hydrogenation of cinnamaldehyde”, Catalysis Science & Technology (2023), DOI: 10.1039/D3CY00289F
- Wijnand Marquart, Michael Claeys, Nico Fischer, “CO2 reduction and C2H6 dehydrogenation over SiO2 supported molybdenum carbide nanoparticles”, Applied Catalysis A: General (2023), 3,DOI: 10.1016/j.apcata.2023.119291
- Motlokoa Khasu, Wijnand Marquart, Patricia J. Kooyman, Charalampos Drivas, Mark Isaacs, Alexander J. Mayer, Sandie E. Dann, Simon Kondrat, Michael Claeys, Nico Fischer “Empowering catalyst supports: a new concept for catalyst design demonstrated in the Fischer-Tropsch synthesis,” Acs Catalysis (2023) 1, 6862 6872, DOI: 10.1021/acscatal.3c00924
- Mohammed J. Islam, Marta Granollers Mesa, Amin Osatiashtiani, Martin J. Taylor, Mark A. Isaacs, Georgios Kyriakou, “The hydrogenation of crotonaldehyde on PdCu single atom alloy catalysts”, Nanomaterials (2023) 13, DOI: 10.3390/nano13081434
- Thulani M. Nyathi, Mohamed I. Fadlalla, Nico Fischer, Andrew P. E. York, Ezra J. Olivier, Emma K. Gibson, Peter P. Wells, Michael Claeys, ”Co3O4/TiO2 catalysts studied in situ during the preferential oxidation of carbon monoxide: the effect of different TiO2 polymorphs”, Catalysis Science & Technology (2023) 151, DOI: 10.1039/D2CY01699K
- Priyanka Verma, Kohsuke Mori, Yasutaka Kuwahara, Maela Manzoli, Sara Morandi, Choji Fukuhara, Robert Raja, Hiromi Yamashita, “Amine functionalization within hierarchically‐porous zeotype framework for plasmonic catalysis over PdAu nanoparticles”, Chemcatchem (2023), DOI: 10.1002/cctc.202201182
- Chitra Sarkar, Ratul Paul, Duy Quang Dao, Shaojun Xu, Rupak Chatterjee, Subhash Chandra Shit, Asim Bhaumik, John Mondal “Unlocking molecular secrets n a monomer-assembly-promoted Zn-metalated catalytic porous organic polymer for light-responsive CO2 insertion”, Acs Applied Materials & Interfaces (2023), DOI: 10.1021/acsami.2c06982
- Man Zhang, Shaojun Xu, Mebrouka Boubeche, Donato Decarolis, Yizhe Huang, Biying Liu, Emma K. Gibson, Xin Li, Yuchen Wang, Huixia Luo, C. Richard A. Catlow , Kai Yan, “Designed TiS 2 nanosheets for efficient electrocatalytic reductive amination of biomass-derived furfurals”, Green Chemistry (2022) 9, DOI: 10.1039/D2GC03234A
- Inns, D.R.; Pei, X.; Zhou, Z.; Irving, D.J.M.; Kondrat, S.A., The influence of phase purity on the stability of Pt/LaAlO3 catalysts in the aqueous phase reforming of glycerol, Materials Today Chemistry, Volume 26, 2022, 101230, DOI: https://doi.org/10.1016/j.mtchem.2022.101230
- Zhang, Jichao; Song, Xuedan; Kang, Liqun; Zhu, Jiexin; Liu, Longxiang; Zhang, Qing; Brett, Dan J.L.; Shearing, Paul R.; Mai, Liqiang; Parkin, Ivan P.; He, Guanjie, Stabilizing efficient structures of superwetting electrocatalysts for enhanced urea oxidation reactions, Chem Catalysis, Volume 2, Issue 11, 2022, Pages 3254-3270,DOI: https://doi.org/10.1016/j.checat.2022.09.023.
- Sun, Hongman; Wang, Chunfen; Sun, Shuzhuang; Lopez, Antonio T.; Wang, Youhe; Zeng, Jingbin; Liu, Zhen; Yan, Zifeng; Parlett, Christopher M.A.; Wu, Chunfei, XAS/DRIFTS/MS spectroscopy for time-resolved operando study of integrated carbon capture and utilisation process, Separation and Purification Technology, Volume 298, 2022, 121622,DOI: https://doi.org/10.1016/j.seppur.2022.121622.
- Hongman Sun, Yu Zhang, Chunfen Wang, Mark A. Isaacs, Ahmed I. Osman, Yehong Wang, David Rooney, Youhe Wang, Zifeng Yan, Christopher M.A. Parlett, Feng Wang, Chunfei Wub, “Integrated carbon capture and utilization: Synergistic catalysis between highly dispersed Ni clusters and ceria oxygen vacancies”, Chemical Engineering Journal 437 (2022) 135394, https://doi.org/10.1016/j.cej.2022.135394
- Yujie Ma, Wanpeng Lu, Xue Han, Yinlin Chen, Ivan da Silva, Daniel Lee, Alena M. Sheveleva, Zi Wang, Jiangnan Li, Weiyao Li, Mengtian Fan, Shaojun Xu, Floriana Tuna, Eric J. L. McInnes, Yongqiang Cheng, Svemir Rudic, Pascal Manuel, Mark D. Frogley, Anibal J. Ramirez-Cuesta, ́ Martin Schröder, and Sihai Yang, “Direct Observation of Ammonia Storage in UiO-66 Incorporating Cu(II) Binding Sites”, J. Am. Chem. Soc. 2022, 144, 8624−8632, https://doi.org/10.1021/jacs.2c00952
- Verma, Priyanka; Potter, Matthew E.; Oakley, Alice E.; Mhembere, Panashe M.; Raja, Robert, Bimetallic PdAu Catalysts within Hierarchically Porous Architectures for Aerobic Oxidation of Benzyl Alcohol, Nanomaterials 2021, 11(2), 350; https://doi.org/10.3390/nano11020350
- Kang, Liqun; Wang, Bolun; Güntner, Andreas T.; Xu, Siyuan; Wan, Xuhao; Liu, Yiyun; Marlow, Sushila; Ren, Yifei; Gianolio, Diego; Tang, Chiu C.; Murzin, Vadim; Asakura, Hiroyuki; He, Qian; Guan, Shaoliang; Velasco-Vélez Juan J.; Pratsinis, Sotiris E.; Guo, Yuzheng; Wang, Feng Ryan, The Electrophilicity of Surface Carbon Species in the Redox Reactions of CuO-CeO2Catalysts, Angew. Chem. Int. Ed. 2021, 60, 14420. DOI:https://doi.org/10.1002/anie.202102570
- Cano, Israel; Weilhard, Andreas; Martin, Carmen; Pinto, Jose; Lodge, Rhys W.; Santos, Ana R.; Rance, Graham A.; Åhlgren, Elina Harriet; Jónsson, Erlendur; Yuan, Jun; Li, Ziyou Y.; Licence, Peter; Khlobystov, Andrei N.; Alves Fernandes, Jesum, Blurring the boundary between homogenous and heterogeneous catalysis using palladium nanoclusters with dynamic surfaces, Nature Communications volume 12, Article number: 4965 (2021), DOI: 10.1038/s41467-021-25263-6
- George F. Tierney, Shahram Alijani, Monik Panchal, Donato Decarolis, Martha Briceno de Gutierrez, Khaled M. H. Mohammed, June Callison, Emma K. Gibson, Paul B. J. Thompson, Paul Collier, Nikolaos Dimitratos, E. Crina Corbos, Frederic Pelletier, Alberto Villa, and Peter P. Wells, “Controlling the Production of Acid Catalyzed Products of Furfural Hydrogenation by Pd/TiO2”, ChemCatChem 2021, 13, 5121–5133, https://doi.org/10.1002/cctc.202101036
- Donald R. Inns, Alexander J. Mayer, Vainius Skukauskas, Thomas E. Davies, June Callison, Simon A. Kondrat, “Evaluating the Activity and Stability of Perovskite LaMO3 Based Pt Catalysts in the Aqueous Phase Reforming of Glycerol”, Topics in Catalysis (2021) 64:992–1009, https://doi.org/10.1007/s11244-021-01449-6
- Zhao, Siyu; Xie, Ruikuan; Kang, Liqun; Yang, Manni; He, Xingyu; Li, Wenyao; Wang, Ryan; Brett, Dan J. L.; He, Guanjie; Chai, Guoliang; Parkin, Ivan P., “Enhancing Hydrogen Evolution Electrocatalytic Performance in Neutral Media via Nitrogen and Iron Phosphide Interactions”, Small Sci. 2100032, https://doi.org/10.1002/smsc.202100032
- Wijnand Marquart, Shaine Raseale, Gonzalo Prieto, Anna Zimina, Bidyut Bikash Sarma, Jan-Dierk Grunwaldt, Michael Claeys, and Nico Fischer, “CO2 Reduction over Mo2CBased Catalysts”, ACS Catalysis, 2021, 11, 3, 1624–1639, https://doi.org/10.1021/acscatal.0c05019
- Mitchell, Claire E.; Terranova, Umberto; Beale, Andrew M.; Jones, Wilm; Morgan, David J.; Sankar, Meenakshisundaram; de Leeuw, Nora H. “A surface oxidised Fe–S catalyst for the liquid phase hydrogenation of CO2”, Catal. Sci. Technol., 2021, 11, 779-784, https://doi.org/10.1039/D0CY01779E
- Antonis Vamvakeros, Dorota Matras, Simon D.M. Jacques, Marco di Michiel, Vesna Middelkoop, Peixi Cong, Stephen W.T. Price, Craig L. Bull, Pierre Senecal, Andrew M. Beale, “Real-time tomographic diffraction imaging of catalytic membrane reactors for the oxidative coupling of methane”, Catalysis Today, Volume 364, 2021, Pages 242-255, https://doi.org/10.1016/j.cattod.2020.05.045.
- Islam, Mohammed J., Granollers Mesa, Marta, Osatiashtiani, Amin, Manayil, Jinesh C., Isaacs, Mark A., Taylor, Martin J., Tsatsos, Sotirios, Kyriakou, Georgios, “PdCu single atom alloys supported on alumina for the selective hydrogenation of furfural”, Applied Catalysis B: Environmental, Volume 299, 2021, 120652, https://doi.org/10.1016/j.apcatb.2021.120652.
- Huang, Haoliang, Blackman, Oliver F., Celorrio, Veronica, Russell, Andrea E., “Isolating the contributions of surface Sn atoms in the bifunctional behaviour of PtSn CO oxidation electrocatalysts”, Electrochimica Acta, Volume 390, 2021, 138811, https://doi.org/10.1016/j.electacta.2021.138811.
- Islam, Mohammed J., Granollers Mesa, Marta, Osatiashtiani, Amin, Taylor, Martin J., Manayil, Jinesh C., Parlett, Christopher M.A., Isaacs, Mark A., Kyriakou, Georgios, “The effect of metal precursor on copper phase dispersion and nanoparticle formation for the catalytic transformations of furfural”, Applied Catalysis B: Environmental, Volume 273, 2020, 119062, https://doi.org/10.1016/j.apcatb.2020.119062.
- Ledendecker, Marc, Pizzutilo, Enrico, Malta, Grazia, Fortunato, Guilherme V., Mayrhofer, Karl J. J., Hutchings, Graham J., Freakley, Simon J., “Isolated Pd Sites as Selective Catalysts for Electrochemical and Direct Hydrogen Peroxide Synthesis”, ACS Catal. 2020, 10, 10, 5928–5938, https://doi.org/10.1021/acscatal.0c01305
- Liu, Yanan; Fu, Fengzhi; McCue, Alan; Jones, Wilm; Rao, Deming; Feng, Junting; He, Yufei; Li, Dianqing, Adsorbate-Induced Structural Evolution of Pd Catalyst for Selective Hydrogenation of Acetylene, CS Catal. 2020, 10, 24, 15048–15059, 2020, https://doi.org/10.1021/acscatal.0c03897
- Potter, Matthew E., Ross, Cameron P., Gianolio, Diego, Rios, Ramon, Raja, Robert, “Cobalt-containing zeolitic imidazole frameworks for C–H activation using visible-light redox photocatalysis”, Catal. Sci. Technol., 2020, 10, 7262-7269, DOI: 10.1039/D0CY01061H.
- Phuoc Hoang Ho, Giancosimo Sanghez de Luna, Saverio Angelucci, Andrea Canciani, Wilm Jones, Donato Decarolis, Francesca Ospitali, Elena Rodriguez Aguado, Enrique Rodríguez-Castellón, Giuseppe Fornasari, Angelo Vaccari, Andrew M. Beale, Patricia Benito, “Understanding structure-activity relationships in highly active La promoted Ni catalysts for CO2 methanation”, Applied Catalysis B: Environmental, Volume 278, 2020, 119256, https://doi.org/10.1016/j.apcatb.2020.119256.
- Kang, Liqun, Wang, Bolun, Thetford, Adam, Wu, Ke, Danaie, Mohsen, He, Qian, Gibson, Emma K., Sun, Ling‐Dong, Asakura, Hiroyuki, Catlow, C. Richard A., Wang, Feng Ryan. “Design, Identification, and Evolution of a Surface Ruthenium(II/III) Single Site for CO Activation”, Angew. Chem. Int. Ed. 2021, 60, 1212., https://doi.org/10.1002/anie.202008370
- Lin, L., Sheveleva, A.M., da Silva, I. et al. “Quantitative production of butenes from biomass-derived γ-valerolactone catalysed by hetero-atomic MFI zeolite.”, Nature Materials (2020), https://doi.org/10.1038/s41563-019-0562-6
- Matthew E Potter, Lauren N. Riley, Alice E. Oakley, Panashe M. Mhembere, June Callison and Robert Raja, “The influence of porosity on nanoparticle formation in hierarchical aluminophosphates”, Beilstein Journal of Nanotechnology, https://dx.doi.org/10.3762/bxiv.2019.35.v1
- Shan Jiang, Harrison J. Cox, Evangelos I. Papaioannou, Chenyang Tang, Huiyu Liu, Billy J. Murdoch, Emma K. Gibson, Ian S. Metcalfe, John S. O. Evans and Simon K. Beaumont, “Shape-persistent porous organic cage supported palladium nanoparticles as heterogeneous catalytic materials”, Nanoscale, https://dx.doi.org/10.1039/C9NR04553H
- Hasliza Bahruji , Norli Abdullah , Scott M. Rogers , Peter P. Wells , C. Richard A. Catlow , Michael Bowker, “Pd local structure and size correlations on the activity of Pd/ TiO2 for photocatalytic reforming of methanol” ,Physical Chem Chem Physics, https://dx.doi.org/10.1039/C9CP00826H
- Samuel D. Cosham, Veronica Celorrio, Alexander N. Kulak and Geoffrey Hyett,”Observation of visible light activated photocatalytic degradation of stearic acid on thin films of tantalum oxynitride synthesized by aerosol assisted chemical vapour deposition”, Dalton Transactions (2019) https://doi.org/10.1039/C8DT04638G
- Stuart A. Bartlett , Emma V. Sackville , Emma K. Gibson , Veronica Celorrio , Peter P. Wells , Maarten Nachtegaal , Stafford W. Sheehan , Ulrich Hintermair “Evidence for tetranuclear bis-μ-oxo cubane species in molecular iridium-based water oxidation catalysts from XAS analysis”, Chemical Communications (2019) https://doi.org/10.1039/C9CC02088H
- Caio V.S. Almeida, Denis S. Ferreira, HaoliangHuang, Ana C. Gaiotti, Giuseppe A. Camara, Andrea E.Russell, Katlin I.B. Eguiluz, Giancarlo R. Salazar-Banda, “Highly active Pt3Rh/C nanoparticles towards ethanolelectrooxidation. Influence of the catalyst structure”, Applied Catalysis B (2019) https://doi.org/10.1016/j.apcatb.2019.04.078
- Moritz Wolf, Emma Kate Gibson, Ezra J. Olivier, Jan H Neethling, C. Richard A. Catlow, Nico Fischer, and Michael Claeys, “Water-induced formation of cobalt-support compounds under simulated high conversion Fischer-Tropsch environment”, ACS Catalysis (2019) https://doi.org/10.1021/acscatal.9b00160
- A. M. Messinis , S. L. J. Luckham , P. P. Wells, D. Gianolio, E. K. Gibson , H. M. O’Brien , H. A. Sparkes, S. A. Davis, J. Callison, D. Elorriaga , O. Hernandez-Fajardo, and R. B. Bedford , “The highly surprising behaviour of diphosphine ligands in iron-catalysed Negishi cross-coupling”, Nature Catalysis (2019), 2, 123.
- Xu, R. et al. Nanoporous carbon: Liquid-free synthesis and geometry dependent catalytic performance Acs Nano (2019) https://doi.org/10.1021/acsnano.8b09399
- M.J. Lawrence, V. Celorrio, X. Shi, Q. Wang, A. Yanson, N.J.E. Adkins, M. Gu, J. Rodríguez-López, P. Rodriguez. Electrochemical Synthesis of Nanostructured Metal-doped Titanates and Investigation of Their Activity as Oxygen Evolution Photoanodes. Aceptted in ACS Applied Energy Materials.


