Methanol to hydrocarbons (MTH) is an important petrochemical process due to its ability to replace conventional fossil fuels (e.g., coal and crude oil) based gasoline, olefins and aromatics production with carbon neutral renewable methanol feedstock, which can be derived from CO2 reduction with H2O. The MTH process can reduce the net carbon emissions from vehicles and polymer (e.g., polypropylene) industries with the existing infrastructure, and hence it can potentially play a key role in the UK’s ambitious target of achieving net zero carbon emissions by 2050 (Fig. 1).
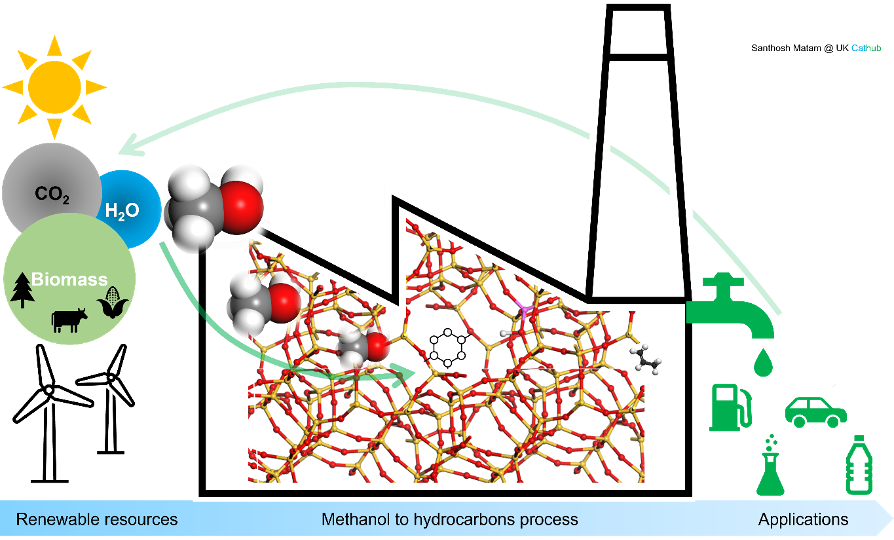
Given the commercial and environmental significance of the process, a great deal of research has been dedicated by the scientific community and industry to better understand the fundamental reaction and deactivation mechanisms and to design and develop zeolite (catalyst that drives the MTH reaction) porous architecture. The Research Complex at Harwell is ideally located within the proximity of neutron and X-ray sources and is well equipped with lasers and other key facilities including computational expertise. This enables us to investigate comprehensively the MTH reaction on zeolites ZSM-5 and SAPO-34 with a wide range of time (ps-minutes), space (nm-μm) and depth (surface-bulk) resolutions. The complementary techniques reveal that:
i) Methanol forms methoxy species (a key intermediate) at the active acidic site of the zeolite
ii) Methoxy species breakaway under certain conditions as propylene and ethylene molecules (olefins), which are the first C-C bond containing molecules formed from C1 methanol molecules
iii) Part of olefins polymerise and cyclise to yield aromatics like alkylated benzenes
iv) Aromatics are the precursors for the zeolite deactivation
v) Computational tools aid to interpret the complex spectroscopic data and to validate results




