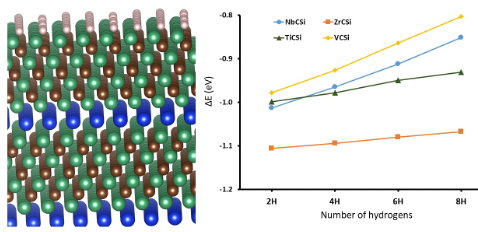
The “MAX”-phase of over 100 ternary carbides/nitrides was first identified in the 1960s.1 The nomenclature is an abbreviation of the general formula of these 2D material ‘Mn+1AXn’, whereby M denotes a transition metal and A/X represent an A group element and carbon/nitrogen respectively .2,3 They also display a very novel combination of properties including high conductivity and extreme resistance to oxidation or heating,4,5 which has led to these materials being labeled as “metallic ceramics”.6 Structurally, the surfaces of these materials strongly resemble the pristine (111) facets of early transition metal carbides (TMCs), which have themselves been shown to be efficient catalysts for the hydrogenation of CO2.7,8 Our group has previously undertaken a detailed in silico study into the catalytic activity of the equivalent TMCs to the MAX-phase material under investigation in this work and found the metal terminated (111) facets to be extremely active for CO2 reduction.9 The choice of the transition metal components was partly informed by this study and partly by a systematic screening of the bulk and surface properties of a diverse array of carbides.10 Therefore, this Faraday Discussion will present the results from a computational study into the bulk and catalytic properties of Ti4SiC3, V4SiC3, Nb4SiC3 & Zr4SiC3 and compare these properties to those already obtained for TiC, VC, NbC & ZrC. We will show that the addition of an intestinal silicon layer into the bulk of these material increases the lattice paraments beyond those observe for the corresponding carbides. We also show that the pristine surfaces of the MAX-phase materials are much more active towards CO2, H2, H2O and OH. However, our results demonstrate that pristine surfaces are not likely to be present in an oxygenating environment. Instead, the modelling predicts all surfaces will oxidise and barriers for CO2 reduction will increase in the presence of H2O, O2 or OH.
Matthew Quesne (Cardiff)
Matthew G. Quesne,1,2* Nora H. de Leeuw,1,3 C. Richard A. Catlow1,2
1School of Chemistry, Cardiff University, Main Building, Park Place, Cardiff, CF10 3AT, UK
2UK Catalysis Hub, Research Complex at Harwell, STFC Rutherford Appleton Laboratory, Didcot, OX11 0FA, UK,
3School of Chemistry, University of Leeds, Leeds LS2 9JT, U.K*Corresponding author
Acknowledgments
The authors acknowledge funding from the EPSRC under the grant no. EP/N009533/1, a multi-disciplinary approach to generating low carbon fuels. The UK Catalysis Hub is thanked for resources and support provided via membership of the UK Catalysis Hub Consortium and funded by EPSRC (Grants EP/R026815/1, EP/ K014854/1, and EP/M013219/1).
References
[1] W. Jeitschko, H. Nowotny and F. Benesovsky, Monatshefte für Chemie, 1963, 94, 672–676.
[2] Z. M. Sun, Int. Mater. Rev., 2011, 56, 143–166.
[3] M. W. Barsoum and T. El-Raghy, Am. Sci., 2001, 89, 334–343.
[ 4] P. Eklund, M. Beckers, U. Jansson, H. Högberg and L. Hultman, Thin Solid Films, 2010, 518, 1851–1878.
[5] T. El-Raghy, A. Zavaliangos, M. W. Barsoum and S. R. Kalidindi, J. Am. Ceram. Soc., 2005, 80, 513–516.
[6] Z. M. Sun, H. Hashimoto, Z. F. Zhang, S. L. Yang and S. Tada, Mater. Trans., 2006, 47, 170–174.
[7] M. D. Porosoff, S. Kattel, W. Li, P. Liu and J. G. Chen, Chem. Commun., 2015, 51, 6988–6991.
[8] C. Kunkel, F. Viñes and F. Illas, Energy Environ. Sci., 2016, 9, 141–144.
[9] M. G. Quesne, A. Roldan, N. H. de Leeuw and C. R. A. Catlow, Phys. Chem. Chem. Phys., 2019, 21, 10750–10760.
[10] M. G. Quesne, A. Roldan, N. H. De Leeuw and C. R. A. Catlow, Phys. Chem. Chem. Phys., 2018, 20, 6905–6916.




