We are working on developing electrodeposition methods for directly synthesizing Ni-based catalysts on porous carbon transport layers (PTLs) for application in zero gap AEM electrolysers.
Electrodeposition is a useful technique because:
(i) Conformal coatings can be deposited onto geometrically complex substrates;
(ii) Cheap substrates, such as carbon, can be used as they will be protected from electrochemical degradation by the overlying catalysts;
(iii) Ni is carcinogenic and poses health risks if deposited using conventional electrode fabrication methods like spray painting, which can cause Ni particles to become airborne.
We are systematically investigating electrodeposition of pure Ni and NiMo alloys, including:
• An in-depth study of the electrodeposition of pure Ni, looking into the influence of solution pH and applied electrode potentials. The study involves a combination of small-scale potentiostatic and potentiodynamic experiments, as well as electrochemical quartz crystal microbalance (EQCM) analysis to determine the Ni2+ reduction efficiency (usually < 100 % due to concurrent H2 evolution).
• For NiMo, the research is geared towards enhancing the Mo content in the NiMo alloy. This can be achieved by varying the complexing agents and operating pH and by employing the Ni-Mo-H2O potential-pH diagram to guide the appropriate operating conditions. EQCM is also being utilized to determine the optimal plating potential and operating conditions, which are more complex than in the case of pure Ni.
• To scale up the electrodeposition process onto carbon cloths, a dedicated flow reactor is being employed, aiming to achieve conformal Ni or NiMo deposits (Fig 1), without forming bulk oxide phases. Effective mass transport of reactants to carbon fibre surfaces within the cloth is achieved through induced electrolyte flow.
• Attempts are being made to translate the results obtained on small-scale Au-coated EQCMs with those obtained on 10 cm x 5 cm carbon cloth substrates in a bench-scale flow reactor (though with limited success).
• Ex situ surface characterization techniques are used to determine deposit compositions: scanning electron microscopy (SEM) imaging, energy dispersive X-ray (EDS) elemental characterisation and X-ray photoelectron spectroscopy (XPS). This enables systematic correlation of electrodeposit composition with electrodeposition conditions. It also allows the visualization of deposit morphology and extent of carbon fibre coverage.
• The electro-coated carbon cloth electrodes are utilized as cathodes in a bench-scale zero gap AEM electrolyser, with Pt/C gas diffusion electrodes (GDEs) serving as a benchmark. Notable enhancements of NiMo cathodes relative to Ni have been observed thus far; the polarization curve of the optimally-prepared NiMo electrode displayed 78% better performance when compared to pure Ni catalyst.
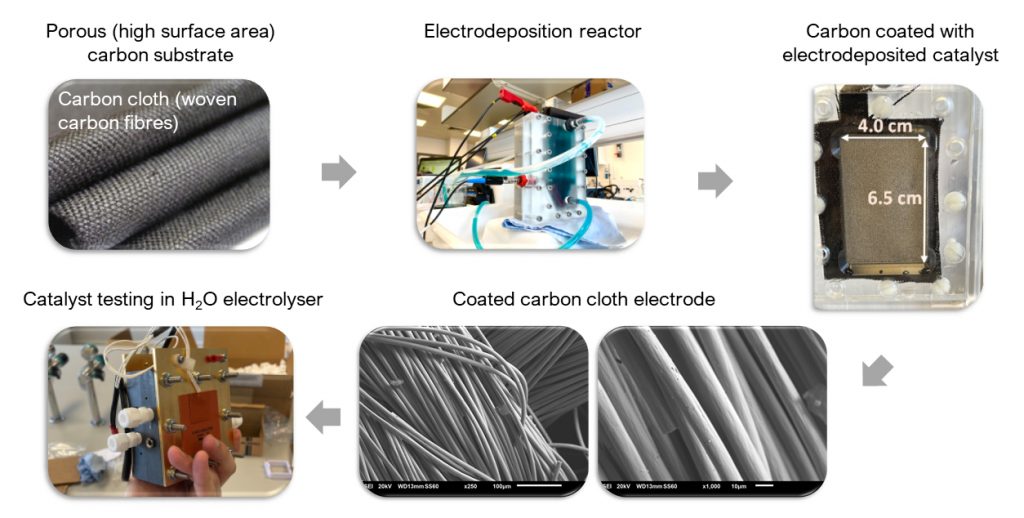
Figure 1
Biography

Anna Hankin is a Lecturer in the Department of Chemical Engineering at Imperial College London (ICL). Her principal interests and expertise are in the science and engineering of electrochemical energy conversion, CO2 reduction and separation processes for industrial effluent treatment and material recycling. After obtaining her MSci degree in Physics at ICL in 2007, she moved to the Department of Chemical Engineering to carry out PhD studies in electrochemical wastewater treatment through heavy metal recovery. She subsequently conducted multiple postdoctoral research projects in the same department, including in photoelectrochemical solar fuel production, waste management by electrochemical treatment of waste streams and valorisation of CO2 via conversion into fuels. Academic research projects in her group are aimed at solving industrial problems through both experimental and numerical modelling investigations. More information can be found on her group website: https://www.imperial.ac.uk/electrochemical-systems-laboratory/




