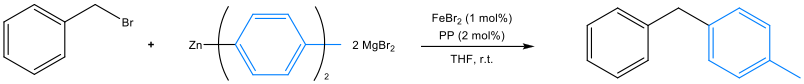
EAM Catalysed C-C and Beyond- Homogeneous cross-coupling reactions based on platinum group metals (PGMs), are widely used in organic synthesis due to their robustness and usefulness. However, their widespread use is limited due to their high cost, detrimental environmental impact upon extraction, and their relatively high toxicity, with subsequent stringent regulations in areas such as the pharmaceutical industry. Current interests seek to replace catalysts based in PGMs, with Earth-abundant elements (EAMs), due to their comparatively high abundance, greater sustainability, reduced cost and considerably lower toxicity. In line with this, previous work in the Bedford group, has involved the use of bidentate phosphines in the iron-catalysed Negishi cross-coupling of benzyl bromide and Zn(p-tol)2 / 2MgBr2 (Scheme 1). From this, it has been determined that the successful Csp3-Csp2 coupling, is highly dependent on the auxiliary phosphine employed.
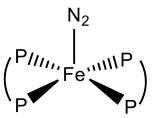
Towards the Reductive Scission of N2 by Iron Complexes- Particularly of stabilised iron centres, and taking into account that there is great interest in the design of synthetic systems that are capable of reducing N2 to NH3, under mild conditions [Nitrogen Fixation]. Achieving this is a challenging endeavour due to the poor donor/acceptor capabilities of these molecule (poor σ-donor & poor π-acceptor). Despite this, there is an increasing number of iron-dinitrogen species (Figure 1). These include [Fe(dppe)2(N2)], [Fe(dmpe)2(N2)] and [Fe(depe)2(N2)] {dppe= 1,2-bis(diisopropylphosphino)propane, dmpe= 1,2-bis(dimethylphosphino)ethane and depe= 1,2-bis(diethylphosphino)ethane).[1–3]
With this in mind, we have focused our efforts on the formation of coordination species, of the form FeX2·(PP)2; X= Cl or Br. As such, and following reported protocols targeting the synthesis of six-coordinate bisphosphine species; QxPPh2 was reacted with FeBr2 (2:1).[4] Single XRD studies have been carried out, furtherly authenticating the aforementioned structure [FeCl2(QxPPh2)2.
Subsequently, we have carried out studies aiming to determine the optimum reducing agent, targeting their corresponding Fe(I)/Fe(0)-N2 species. Currently we are studying the product of reduction/halide scavenging of FeCl2(dpbz)2. From this, it has been possible to identify formation of a sole species.[5–7] Based on our NMR determinations, we suspect a deviation from an idealised square pyramidal conformation in [Fe(dpbz)2]-N2.
PI(s): Prof. Robin B. Bedford
PDRA: Dr. Roberto Nolla Saltiel
References
[1] J. L. Crossland, D. R. Tyler, Coord. Chem. Rev. 2010, 254, 1883–1894.
[2] R. A. Cable, M. Green, R. E. Mackenzie, P. L. Timms, T. W. Turney, J. Chem. Soc. Dalton Trans. 1976, 270–271.
[3] D. E. Prokopchuk, E. S. Wiedner, E. D. Walter, C. V. Popescu, N. A. Piro, W. S. Kassel, R. M. Bullock, M. T. Mock, J. Am. Chem. Soc.2017, 139, 9291–9301.
[4] A. M. Messinis, S. L. J. Luckham, P. P. Wells, H. A. Sparkes, S. A. Davis, J. Callison, O. Hernandez-Fajardo, D. Elorriaga, E. K. Gibson, R. B. Bedford, et al., Nat. Catal. 2019, 2, 123–133.
[5] L. R. Doyle, P. J. Hill, G. G. Wildgoose, A. E. Ashley, Dalton Trans. 2016, 45, 7550–7554.
[6] S. Komiya, M. Akita, A. Yoza, N. Kasuga, A. Fukuoka, Y. Kai, J. Chem. Soc. Chem. Commun. 1993, 205, 787–788.
[7] J. D. Gilbertson, N. K. Szymczak, D. R. Tyler, J. Am. Chem. Soc. 2005, 127, 10184–10185.




