Introduction. Electrolysis is one of the most promising methods for the production of hydrogen via water splitting. However, the sluggish oxygen evolution reaction (OER) leads to cell overpotentials higher than the thermodynamic value, 1.23 VRHE, with energy losses up to 53%.[1] While iridium oxide, IrO2, has proven to be one of the most promising materials to achieve OER in acidic media, obtaining catalysts with lower overpotentials is still necessary, with a focus on the fine balance between activity and stability. Synthesising Ir(O)x(OH)y in the presence of alkali metal salts has been shown to affect the morphology and composition of the catalyst surface, with an effect on its electrocatalytic properties.[2] We have developed a facile synthesis method to produce nanocrystalline lithium iridate, Li2IrO3. This material has shown remarkable activity and stability towards OER, with the former comparable to that of amorphous Ir(O)x(OH)y, breaking the delicate relationship between high activity and low stability.
Experimental/Methodology. Our novel approach allows for the synthesis of nanocrystalline Li‑intercalated iridium oxide, Li-IrOx, via heat treating amorphous Ir(O)x(OH)y (500°C, 3 hours), previously obtained in the presence of Li2CO3 following a procedure described by Esquius et al.[2] The product is then washed with 1 L cold and 1 L hot water and left to dry in open air. Linear scanning voltammetry (LSV) and chronopotentiometry (CP) are used in a 3‑electrode setup containing 0.1 M HClO4 electrolyte to investigate the electrocatalytic activity and the stability of the catalyst, respectively. The material was characterised using XRD, STEM, operando XAS and XPS. In a similar fashion, the Na- and K-containing analogues have been synthesised and characterised revealing a trend in the electrocatalytic performance.
Results and discussion. Following the synthesis of the amorphous Ir(O)x(OH)y, residual Li+ is shown to suppress the formation of rutile IrO2 upon thermal treatment and leads to a transition to a nanocrystalline Li-layered iridium oxide, whereas the removal of residual Li+ results in the formation of rutile IrO2 (Figure 1a). High resolution aberration corrected transmission electron microscopy shows the lithium‑intercalated iridium oxide is polycrystalline with nanocrystalline domains. The Li-intercalated phase shows electrocatalytic activity comparable to amorphous iridium oxide while exhibiting a remarkable stability, similar to that shown by rutile IrO2 (Figure 1b and 1c). These results suggest the incorporation of metal alkali in the structure of the catalyst leads to improved stability while retaining significant activity towards OER, potentially offering the opportunity to produce highly active and durable electrocatalysts for industial applications.
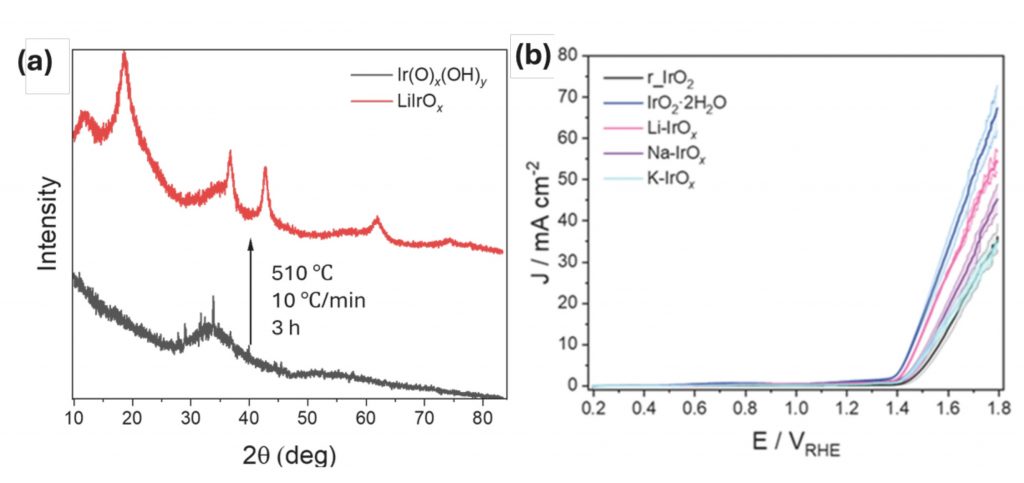
Figure 1. a) XRD pattern (Cu Kα = 1.5046 Å) of synthesised amorphous Ir(O)x(OH)y and LiIrOx. b) LSV (1.2-1.7 VRHE, 5 mV s-1) of IrOx, Li-IrOx, Na-IrOx, K-IrOx and rutile IrO2 showing the activity of the synthesised vs commercially available electrocatalysts.
References
[1] Wang, M.; Wang, Z.; Gong, X.; Guo Z., Renewable and Suistanable Energy Reviews 2014, 29, 573-588
[2] Esquius, J. R.; Morgan D. J.; Spanons I.; Hewes D. G.; Freakley S. J.; Hutchings G. J., ACS Applied Energy Materials 2020, 3, 800-809
M. Falsaperna,1 S. J. Freakley1
1University of Bath, Bath, United Kingdom
*sf756@bath.ac.uk
Biography:

Mario Falsaperna obtained his MSc in Materials Chemistry in 2018 from the University of Catania, Italy, working on MOFs for gas separation. He obtained his PhD in Chemistry from the University of Kent, UK, working on coordination polymers exhibiting magnetocaloric properties and their optimisation for low-temperature magnetic refrigeration, with an interest in their frustrated and low-dimensional magnetic states. In 2022, he joined the Department of Chemistry at the University of Bath as a postdoctoral associate. His work focuses on the development, characterisation and testing of novel alkali metal-containing iridium-based catalysts for water electrolysers.




