As underlined in the COP26, methane as a greenhouse gas is nearly 25 time more potent than CO2 while its reserve is much more than the sum of coal, oil and natural gas. Thus methane conversion not only involves the environmental issue but more importantly is regarded as the most promising pathway for high-value chemical synthesis. However CH4 activation is energy intensive and kinetically very sluggish so that methane activation is regarded as the “holy grail” in the catalytically chemical process [1]. Photocatalysis provides a cost-efficient potential to activation of such small molecule under very mild conditions, while to achieve the potential is a huge challenge [2].
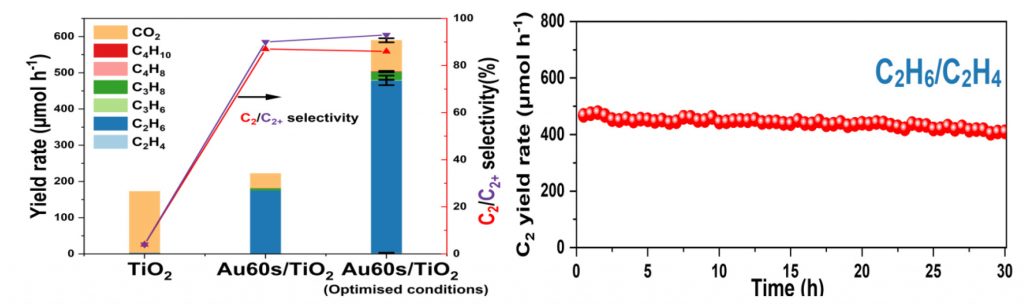 Fig CH4 conversion to C2 by photo-thermo catalysis under very mild condition, left: on different catalysts, right: stability of the Au/TiO2 catalyst.
Fig CH4 conversion to C2 by photo-thermo catalysis under very mild condition, left: on different catalysts, right: stability of the Au/TiO2 catalyst.
Stimulated by our research outcomes on the charge dynamics, which reveal that the low reaction efficiency is due to fast charge recombination and large bandgap of a semiconductor [3,4], together with the recent findings on atomic catalysis [5], we developed novel material strategies for photocatalytic methane conversion. Highly dispersed atomic level iron on TiO2 shows an excellent activity for methane conversion, resulting into ~97% selectivity towards alcohols operated under ambient conditions by a one-step chemical process [6]. The dominating function of the iron species has also been investigated in detail. C1 oxygenates can also be produced with nearly 100% selectivity by oxide photocatalyts due to the synergy between Au and Cu cocatalysts loaded on ZnO [7]. Furthermore, we coupled photons with phonons (the concept of photo-thermo catalysis) to co-drive catalytic methane conversion to C2 over Au loaded TiO2, achieving the benchmark results in this area [8]. Finally for the first time, an intramolecular junction was designed to convert methane to ethanol in a one-step process, which shows the unprecedented yield of ethanol under ambient conditions [9].
References:
- Li, X. Wang, C., Tang, J. Nature Reviews Materials, 2022, 7, 617–632.
- Thangamuthu, L., Ruan, Q., Ohemeng, P.S., Luo, L, Jing, D., Godin, R., Tang, J., Chemical Reviews, 2022, 122 (13), 11778-11829.
- Tang, J. Durrant J. R., Klug, D. R ., J. Am. Chem. Soc., 2008, 130(42) : 13885-13891.
- Miao, T., Wang, C., Xiong, L., Li, X., Xie, X., Tang, J., ACS Catalysis, 2021, 11, 8226-8238.
- Wang, A. Li J., Zhang. T., Nature Review Chemistry, 2018, 2; 65-81.
- Xie, J., Jin, R., Li, A., Bi, Y., Sankar, G., Ma D., Tang, J. Nature Catalysis, 2018, 1: 889-896.
- Luo, L., Gong, Z., Xu, Y., Ma, J., Liu, H., Xing, J., Tang, J., Journal of the American Chemical Society, 2022, 144, 2, 740–750.
- Li, X., Li, C., Xu, Y., Liu, Q., Bahri, M., Zhang, L., Browning, N.D., Cowan, A.J., Tang, J., Nature Energy, 2023, Doi: 10.1038/s41560-023-01317-5.
- Xie, J., Fu, C., Quesne, M.G., Guo, J., Wang, C., Xiong, L., Windle, C.D., Gadipelli, S., Guo, Z., Huang, W., Catlow, C.R.A.,Tang, J., Nature, 2024, in review.

Prof. Junwang (John) Tang is a Member of the Academy of Europe, a Royal Society Leverhulme Trust Senior Research Fellow, Fellow of the European Academy of Sciences, Fellow of the Royal Society of Chemistry and Fellow of IMMM. He is the Founding Director of Industrial Catalysis Center in the Department of Chemical Engineering and Chair Professor of Materials Chemistry and Catalysis at Tsinghua University, China and an Adjunct Professor at University College London, UK.
Tang’s research interests encompass coupling thermo-catalysis (phonons) with photo-catalysis (photons) for small molecule activation to produce zero-carbon fuels (eg. H2O to H2, N2 to NH3) and valuable chemicals (CO2 to alcohols and CH4 to C2+ hydrocarbons) as well as microwave catalysis (e.g. chemical plastic recycling), together with the investigation of the underlying charge dynamics and kinetics by state-of-the-art spectroscopies, resulting in >250 papers published in Nature Catalysis, Nature Energy, Nature Materials, Nature Reviews Materials, Chemical Reviews, Chem. Soc. Rev., Materials Today, Nature Commu., JACS, Angew Chemie etc. with ~29,000 citations. Prof. Tang has received many awards, the latest of which is the 2022 IChemE Oil and Gas Global Awards, 2021 IChemE Andrew Medal, 2021 the RSC Corday-Morgan Prize and 2021 Royal Society-Leverhulme Trust Senior Research Fellowship etc. He also sits on the Editorial Board of 5 international journals, eg. the Editor of Applied Catalysis B and Associate Editor of Chin. Journal of Catalysis etc.




