The heterogeneously catalysed hydrogenation of nitrobenzene to synthesize aniline is a key reaction in the process chain to produce isocyanates, materials used in the production of polyurethanes. Reflecting the complexity of large-scale isocyanate manufacture, the reaction is typically undertaken within an integrated chemical complex [1]. This scenario then provides the opportunity to utilise the significant quantities of heat associated with the hydrogenation reaction (-443 kJ mol-1 !) to raise super-heated steam, which can then be employed as an energy vector within the particular chemical complex. This exercise in novel sustainable chemical manufacturing requires the hydrogenation reaction to be conducted at elevated temperatures, a condition that can compromise aniline selectivity.
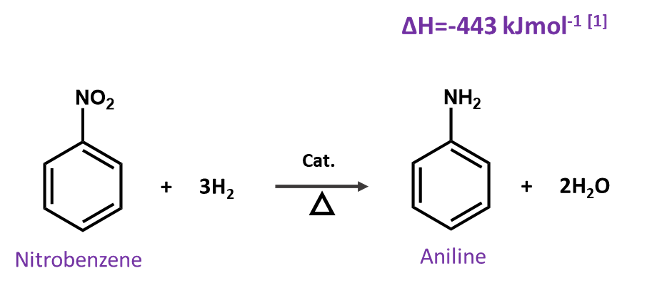
This presentation will describe a collaboration with Huntsman Polyurethanes to develop structure/activity relationships for a series of alumina-supported palladium catalysts specified to deliver high aniline selectivity at elevated temperatures (T>100°C). Importantly, certain catalyst formulations were observed to favour different distributions of by-products, complicating exploitation of the intended step-change in industrial practice.
As previously noted by Robbie Birch, “morphology matters!” [2]. The form and structure of the Pd crystallites was investigated by means of infrared spectroscopy, using carbon monoxide as a probe molecule. First, classical characterisation procedures determined the morphology and energetics of the Pd particles [3]. Second, post-reaction infrared spectroscopic analysis of the catalysts at various stages of the reaction coordinate was insightful in determining how certain facets were modified as a function of time-on-stream. These developments were then combined to understand why certain catalyst formulations exhibited the intended desirable performance. Subsequent infrared spectroscopic studies of nitrobenzene chemisorbed on a representative model catalyst under conditions of constrained hydrogen supply provide insight on the site-selective nature of product and by-product formation.
Prof. David Lennon
School of Chemistry, Joseph Black Building, University of Glasgow, Glasgow G12 8QQ
References
- D. Randall and S. Lee, The Polyurethanes Book, Wiley (2003).
- R. Birch, Sam Thompson Retirement Lectures, University of Glasgow, Glasgow, September 1988.
- T. Lear, R. Marshall, J.A. Lopez-Sanchez, S.D. Jackson, T. M. Klapötke, G. Rupprechter, M. Bäumer, H-J. Freund and D. Lennon, J. Chem. Phys., 123 (2005) 174706.




