Design theme highlights: Advances in Heterogeneous Catalyst Characterisation; combined XAFS/DRIFTS Spectroscopy

Understanding the chemical processes that govern catalytic function is key to developing new and improved catalytic materials. Characterisation methods used to interpret catalyst structure, morphology and chemical properties often examine the catalyst material outside of its operating environment, which can give unrealistic representation of the dynamic catalytic system. Diffuse reflectance infrared spectroscopy (DRIFTS) and X-ray absorption spectroscopy (XAFS) are well established characterisation techniques, using non-invasive radiation that can penetrate reaction medium and thus be used for in situ measurements. In this project, efforts to improve in situ methodology for combined XAFS/DRIFTS spectroscopy aim to elucidate catalyst structure and reactivity under operating conditions.
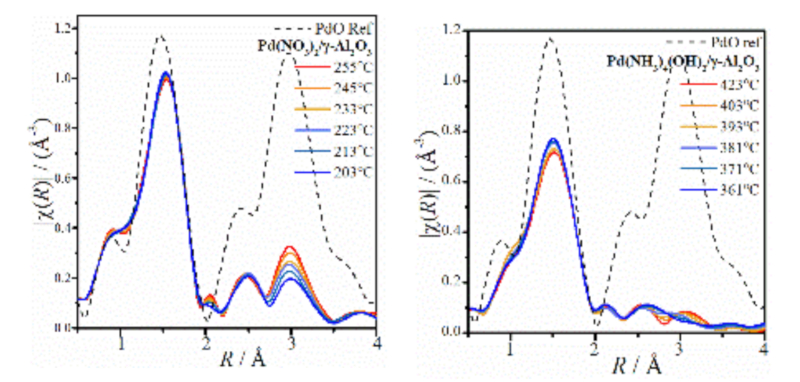
Following the evolution of supported PdO nanoparticles from inorganic molecular precursors; combined XAFS/DRIFTS. A commercial Harrick DRIFTS cell, adapted for simultaneous XAFS was used at B18, Diamond Light Source, in the study of PdO nanoparticle formation from two alternative Pd precursors; Pd(NO3)2 and Pd(NH3)4(OH)2. The aqueous Pd precursor solution (Johnson Matthey) was mixed with γ-Al2O3 (SASOL) in a standard wet impregnation method, dried and loaded into the DRIFTS cell. Both XAFS and DRIFTS were collected continuously during a calcination treatment (500°C, air) with effluent gas analysed by mass spectrometry. Results revealed the nucleation and growth of PdO nanoparticles during the calcination ramp, indicating different pathways from each precursor. For Pd(NO3)2/γ-Al2O3, early association of Pd neighbours was observed before heat treatment; rationalised by the N=O and O-N-O vibrational frequencies of bridging nitrate ligands, and the additional scattering feature in Pd K-edge EXAFS due to neighbouring Pd. Conversely Pd(NH3)4(OH)2 adopts well isolated sites upon impregnation with γ-Al2O3, and thus the formation of PdO nanoparticles is suppressed until much higher temperatures resulting in a catalyst with improved metal dispersion.1
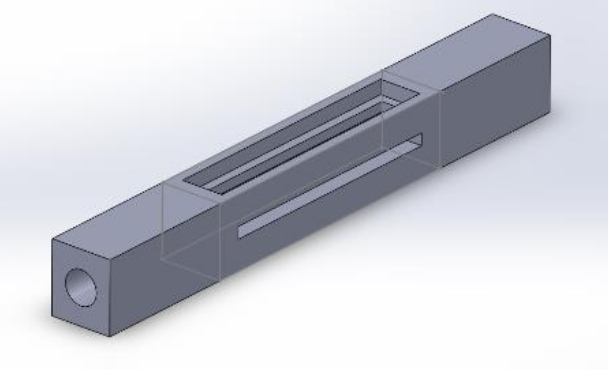
Operando XAFS/DRIFTS cell design. A new cell has been designed to make improvements on the existing set up for combined XAFS/DRIFTS experiments. The new cell is shaped similarly to a flow reactor with linear gas flowing through the fixed catalyst bed and limited dead cell volume. The infrared window positioned on the top face of the reactor is constructed from GaAs and coated with a protective Silicon layer. The reactor body is constructed from Aluminium, able to penetrate high energy X-rays and allow XAFS measurements at multiple positions along the length of the catalyst bed. Several iterations of the cell design have resulted in this final version, which will be commissioned on B18 in January to study bimetallic Pd/Pt/Al2O3 catalysts for CH4 oxidation.
- Dann, E. K.; Gibson, E. K.; Catlow, R. A.; Collier, P.; Eralp Erden, T.; Gianolio, D.; Hardacre, C.; Kroner, A.; Raj, A.; Goguet, A.; Wells, P. P. Chemistry of Materials 2017, 29, 7515.
Author:
Ellie Dann, iCASE, UK Catalysis Hub




