Joint Diamond/UK Catalysis Hub studentship: Novel shell-core catalysts of vanadia on haematite (VOx/Fe2O3) for use in methanol selective oxidation to formaldehyde

Over the past year, we have been investigating novel shell-core catalysts of vanadia on haematite (VOx/Fe2O3) for use in methanol selective oxidation to formaldehyde. The shell-core motif has been well explored with molybdena on haematite (MoOx/Fe2O3) catalysts previously with assistance from Diamond, which has afforded significant structural insight and support during the exploration of our novel VOx/Fe2O3 catalysts. Our intentions in exploring vanadia instead of molybdena were to gauge the applicability of alternative metal oxides as shell materials (in this case vanadia) with respect to methanol oxidation and to examine any potential differences in catalyst structure or speciation. This is part of a wider project to produce new materials for the oxidative dehydrogenation of methanol, and relates to the future development of a ‘green’ methanol economy, based on the use of sustainable hydrogen for synthesis. This could then also be a ‘sink’ process for removal of CO2 from the atmosphere.
In temperature programmed desorption (TPD) studies, high selectivity to formaldehyde is observed with CO as the other main product: crucially, no CO2 is observed in TPD. Fe2O3 is an efficient combustor of methanol; hence any exposed core Fe2O3 will afford CO2 in TPD. Since none is observed, we can assume that shell-core segregation has occurred.
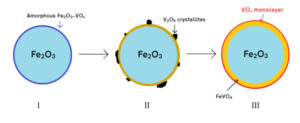
We noted similar structural trends for VOx catalysts to those seen for MoOx catalysts, which is highly encouraging. At a notional one monolayer (ML) of VOx in the shell, we suggest that the shell comprises only VOx; at higher ML loadings (e.g. 3 ML) only the outermost layer consists of VOx, while excess vanadia is incorporated into a sandwich layer of FeVO4 between the haematite core and the VOx surface layer. We can observe the sandwich layer by Raman spectroscopy, in which the relative intensity of the FeVO4 peaks increase with greater ML coverage, although the outermost layer is not visible due to limited dimensionality. High temperatures are required to spread the shell material sufficiently across the haematite to inhibit direct core participation in the catalysis. This strongly resembles the behaviour exhibited by MoOx/Fe2O3 catalysts, in which sandwich layers of iron molybdate are formed above 1 ML and under similar calcination conditions.
Thanks to the BAG time scheme at Diamond, we secured access to beamline B18 to undertake X-ray absorption spectroscopy (XAS) measurements at the V K-edge of our novel VOx/Fe2O3 catalysts. XAS permits the determination of oxidation states and local structure, but is an averaging technique; however, since we know that all V resides in the surface shell layers, all peaks originate from surface species. Following the expected structural behaviour, we expected similarity to FeVO4 to increase with ML coverage: this was indeed observed, most noticeably for the pre-edge feature in the XANES region.
Small differences as seen here are indicative not of oxidation state changes, but small alterations to structural geometry. We suggest that for all cases, the outermost VOx layer contains elongated V-O bonds, distorting the tetrahedral structure, resulting in the skewed 1 ML peak position; for greater ML coverage, however, more FeVO4 is present, which reduces the relative contribution of the VOx layer to the pre-edge peak position. We will be working with our Diamond colleagues on structural modelling and more detailed analysis of the data, especially the XAFS, in the coming months. Additionally we have beamtime scheduled soon to conduct in situ measurements to probe catalyst behaviour, and to assess their applicability to other oxidation reactions of interest.
Author:
Pip Hellier, Cardiff University