Chemical Transformation Theme highlights: Encapsulation of an organometallic cationic catalyst by direct exchange into an anionic MOF
The Chemical Transformations theme of the UK Catalysis Hub has undertaken a wide range of projects broadly concerned with promoting the sustainability and prosperity of the UK manufacturing base by contributing fundamental catalytic science to bulk and fine chemicals synthesis, polymer and pharmaceutical manufacture. One key project highlighting the breadth of multidisciplinary catalytic science taking place within the theme has been encapsulation of homogeneous organometallic catalysts inside the pores of Metal-Organic Frameworks (MOFs). Researchers working in the Catalysis in Confined Environments project of the UK Catalysis Hub have successfully encapsulated a well-defined organometallic catalyst inside the pores of a MOF by cation exchange. This simple approach permits the encapsulation of the whole transition metal catalyst in one step without the necessity to modify its coordination environment.
MOFs are crystalline and porous materials built from metal-based nodes and organic linkers interconnected via strong coordination bonds. MOFs show permanent porosity with pore diameters spanning from 3 to 30 Å. In addition, the shape and chemical composition of the pores can be pre-designed. Therefore, MOFs have emerged as promising solid-state supports (hosts) for the encapsulation of transition metal (TM) organometallic catalysts (guests), leading to new [TM catalyst]@MOF materials. Such hybrid systems could combine the benefits of both homogenous (high reactivity and selectivity, fine-tuning of the catalytically active site) and heterogeneous (stability, recycling, easy separation of products) systems. Employment of MOFs as hosts provides a well-defined environment compared to traditional mesoporous materials like aluminosilicates (lack of crystallinity) and metal oxides (lack of ordered porosity and crystallinity). Moreover, their larger pore size allows for the encapsulation of quite bulky TM catalysts compared to zeolites (smaller pore size) which are currently used in various industrial applications.
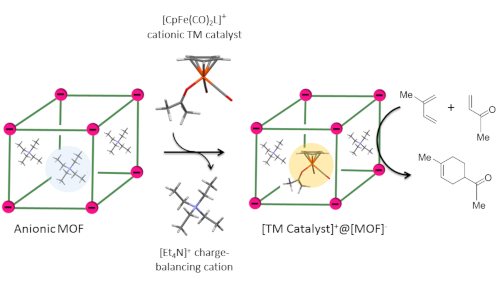
Researchers from the University of Liverpool, University of Oxford, University of Sheffield and Imperial College have developed a new reliable and scalable protocol for the encapsulation of positively charged organometallic TM catalysts inside the pores of anionic MOFs via cation exchange leading to a hybrid between well-defined homogeneous and supported heterogeneous catalysts. This methodology allows for the encapsulation of the TM catalyst as a whole in one step without perturbing the catalyst’s coordination sphere. The cationic TM catalyst is firmly held inside the pores of the anionic host via relatively strong electrostatic interactions and the degree of exchange can be controlled by simply adjusting the stoichiometry of the cation exchange reaction. Encapsulation is expected to spatially isolate and thus protect the catalytic species from deactivation via dimerisation which can be a common deactivation pathway in homogeneous systems. It also potentially allows for simple separation of catalyst and products by filtration, while retaining (or improving) the activity seen in the pure homogenous system.
Proof of principle was provided by encapsulating the Lewis acidic [CpFe(CO)2]+ TM catalyst inside the pores of an indium based [In3(BTC)4]– anionic MOF, forming [CpFe(CO)2]+@[In3BTC4]–. Rigorous characterisation of this new hybrid material conclusively demonstrated that the cationic catalyst is encapsulated in one step and remains intact after encapsulation. The heterogenised catalyst was benchmarked in a Diels-Alder reaction. Comparison with the homogeneous counterpart revealed that the catalyst’s lifetime is prolonged and it can be easily separated from products and recycled. The group is currently investigating other commercially available positively charged TM catalysts in combination with different anionic MOFs in the anticipation that these new hybrid systems will show increased activity, stability and selectivity enabled by the confined and chemically tailored environment of the MOF host.
Read more:
For more details read the full paper in Chemical Science – http://dx.doi.org/10.1039/C5SC03494A
Author:
Dr Alex Grigoropoulos, Research Associate Prof Rosseinsky’s Group, The University of Liverpool, Department of Chemistry




