To achieve net zero, captured carbon dioxide must be efficiently utilised and recycled. A research team led by Dr Simon Kondrat (Loughborough University) and the UK Catalysis Hub are developing robust heterogeneous catalysts to directly produce methanol from amine captured carbon dioxide, which in the process regenerates the original amine. Using a theory led approach, facilitated by the expertise at the Research Complex at Harwell and Diamond Light Source, key properties of the catalyst are identified to assist in its design and characterisation. Successful catalysts could facilitate decentralised utilisation of CO2 and maximise energy savings though limiting CO2 storage and transportation.
Summary: Using a modelling led approach we are designing novel single-site heterogeneous catalysts for the single step hydrogenation of amine captured CO2 to produce methanol and regenerate the amine. The project, as part of the UK Catalysis Hub, has identified key properties and transition states of current homogeneous catalysts and uses this knowledge to develop new heterogeneous catalysts.
The challenge: The drive for net zero CO2 requires new technologies that, (a) produce no CO2 emissions and/or (b) effectively utilise CO2 produced in processes. Ideally, we can recycle emitted CO2 for use in chemical processes. Synthesis of methanol from the hydrogenation of captured CO2 with H2 produced from excess renewable energy is such a CO2 utilisation stratergy. Yet, separation (a high-energy thermal step in commercial amine capture technologies), concentration and transportation of captured CO2 limit application. An exciting prospect is direct catalytic methanol production from captured CO2 that simultaneously regenerates the amine capture technology.
Homogeneous Ru pincer complex catalysts facilitate this reaction, but selectivity issues, deactivation and processing challenges call for the development of a robust heterogeneous equivalent. To date, no heterogeneous catalyst can turnover amine captured CO2 to methanol. In addition, selectivity to methanol is highly dependent on the amine used.
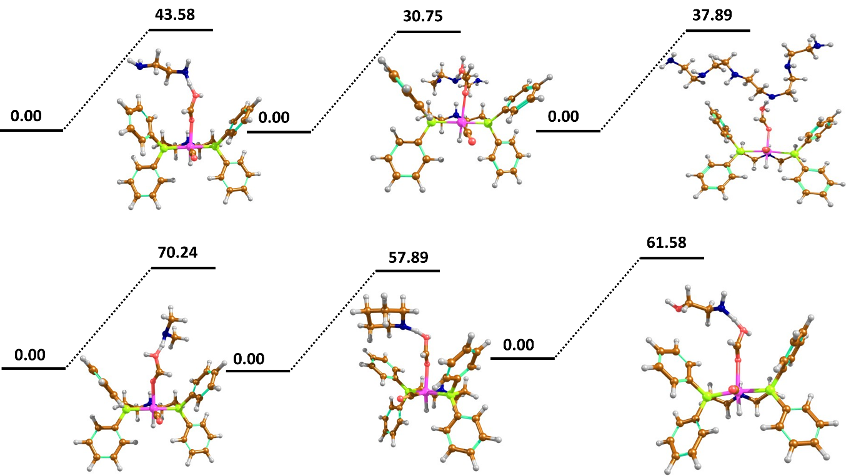
Results: Our central aim is the ambitious design of a heterogeneous direct methanol synthesis catalyst, via key insights provided from understanding the homogeneous catalyst. In this regard we have used density functional theory (DFT) techniques to rationalise varying selectivity of CO2 hydrogenation products catalysed by homogeneous catalysts (Fig 1). Analysis highlights the formic acid to formamide step as key to reaction mechanism bifurcation, with inaccessible high barriers observed when using primary amines. Further, the insights gained suggest heterogenous alternatives require the prominent NH group on the pincer ligand, which stabilises that first CO2 hydrogenation barrier to formate. We also observe a strongly bonding molecular orbital between axially coordinated hydrogens that is important in shuttling electron density along the H-Ru-H axis during the same format producing step.
We are designing polymer matrixes, where essential Ru ligand backbone moieties (as determined from theory) are incorporated into the polymer, to produce heterogeneous catalysts will equivalent Ru centres to successful homogeneous catalysts.