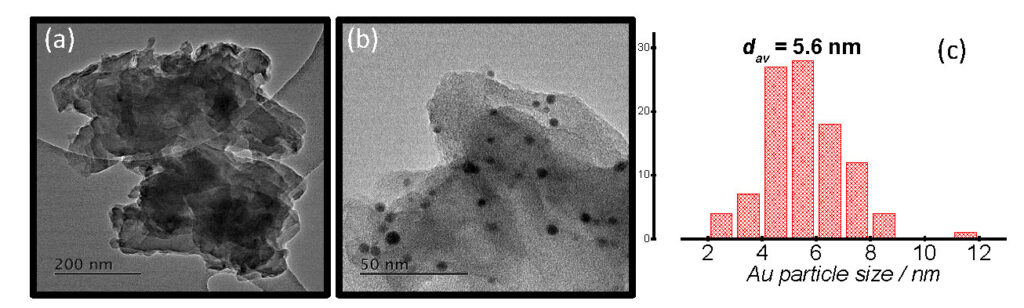
Shale gas, an unconventional natural gas resource, offers an opportunity to access a relatively clean fossil fuel, and could potentially lead to energy independence for some countries [1]. Notably, the extraction of shale gas generates large volumes of produced water estimated to be in the range ~15 000 – 23 000 m3 per well. The management of produced waters generated from hydraulic fracturing (fracking) operations poses environmental and human health challenges. Treatment of the hypersaline produced waters prior to reuse or discharge is nontrivial because the complicated matrix typically detected. In this presentation, we report on the development of energy-efficient photocatalysts for the destruction of organics in simulated produced waters. Optimization studies of the experimental protocol enabled the synthesis of highly porous graphitic carbon nitride support material (~60 m2/g) relative to bulk g-C3N4 (~10 m2/g). Decoration of TiO2 and the porous g-C3N4 supports with plasmonic nanoparticles was achieved by sol immobilization and the modified impregnation techniques (Fig. 1). The efficiency of M/TiO2 and M/g-C3N4 catalysts (M = Au, Pd, Au-Pd, Ru) to completely photo-oxidise organics (initial substrate and reaction intermediates) in such a complex water matrix was monitored by HPLC and UV-Vis spectroscopy. In the brine-free solutions, destruction of the organics proceeded at high reaction rates until complete and was shown to be influenced by the catalyst composition. In the presence of brines, destruction of organics was complex and depended on the concentrations of both the organics (initial substrate, intermediates) and the brine solutions. We have also performed these experiments under pressurised conditions (up to 3 bar O2) to evaluate the influence of oxygen solubility.
Reference
[1] R. D. Vidic, S. L. Brantley, J. M. Vandenbossche, D. Yoxtheimer, J. D. Abad, Science, 2013, 340, 1235009.
Mbongiseni W. Dlaminia, Samuel Pattissona, Philip R. Daviesa, Graham J. Hutchingsa, Christopher Hardacreb
aCardiff Catalysis Institute, School of Chemistry, Cardiff University, Park Place, Cardiff, UK
bSchool of Chemical Engineering and Analytical Science, University of Manchester, Manchester, UK
Watch a recording of the talk below:
Biography

Dr William M. Dlamini completed his PhD (2016) in the School of Chemistry, University of the Witwatersrand, South Africa, working on a project entitled; “Spherical carbons as model supports for Fe, Co and Fe-Co Fischer-Tropsch catalysts”. The project was funded by the Centre of Excellence in Catalysis (c*change) and was done in collaboration with the University of Cape Town. Dlamini then did his Postdoctoral Fellowship with Prof. Neil Coville (2017 – 2018) at the same institution, focusing on the preparation multifunctional core-shell catalysts aimed at a tandem process involving the synthesis of hydrocarbons and their secondary hydrocracking on other active sites of the same catalyst. At present, William is based at Cardiff University working with Prof. Graham Hutchings, Prof. Phil Davis and Prof. Chris Hardacre (Manchester), on a project entitled “High salinity resistant catalysts for the destruction of organics in produced waters”. This project aims to advance the understanding of solardriven wastewater treatment protocols by studying them under rather harsh reaction conditions.




