Introduction
A catalyst is, by definition, a substance that accelerates a reaction but is not consumed or changed. Whereas this is generally true when looking at the start and the end of the reaction, it might not be true during the reaction, since catalysts may change dynamically according to the reaction environment. To understand these changes operando spectroscopy measurements, which combines spectroscopic or diffraction techniques with simultaneous analysis of effluent gases, have been developed and has led to important discoveries in the world of catalysis1. However, since concentration and temperature gradients are present along a plug-flow reactors, the commonly employed single point spectroscopic measurements might not be able to obtain the much-sought insights and including a spatial component in their measurements becomes necessary2,3. To counteract this issue we used the Spaci-FB technique3, which allows to collect spatially resolved XAFS and concurrently monitor the gas-phase concentrations as well as the temperatures along a reactor bed. This method was applied to the selective catalytic oxidation of NH3, an environmentally important reaction which is needed to abate the unwanted NH3 slip resulting from the selective catalytic reduction of NOx compounds. In our recent work5, we reported on a unique structure/selectivity during the NH3-SCO, with different phases forming during the reaction process, each affecting the selectivity of the catalyst. By obtaining spatially resolved measurement it was possible to profile the catalyst properties along a fixed catalytic bed and clarify the relation between structural properties in terms of Pd speciation to selectivity in the NH3-SCO.
Materials and Methods
A 1.5 wt % Pd/γ-Al2O3 was prepared by wet impregnation of an acidified solution of palladium nitrate onto a γ-Al2O3 support, followed by drying at 100 °C and calcination at 500 °C for 2 h. The operando experiments were performed at the Swiss light source at Pd K edge (24.35 keV), using a QEXAFS Si (311) monochromator in transmission mode. Typically, the catalyst was loaded in a quartz reactor within the Spaci-FB system and the capillary gas sampling apertures, the thermocouple and the X-ray beam were aligned to ensure that coincident measurements were conducted at the same axial point in the catalysis bed. The experiment procedure consisted in a pre-reduction at 400 °C using 5% H2 in He, cooling down to 100 °C in He followed by admission of the reactants mixture (0.5% NH3, 2.5% O2 and 97% He). After steady state had been reached, XAFS spectra collection, gas composition analysis and temperature measurements were performed at 11 discrete axial positions, with position 0 being the inlet. The same procedure was employed after raising the reaction temperature to 175, 300 and 400 °C.
Results and Discussion
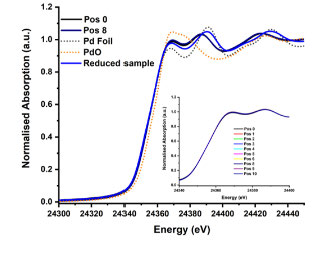
Figure 1 reports the XANES of the sample at 100 °C under reaction conditions. Formation of PdNx can be seen, defined by the higher intensity of the white line at 24368 eV and the shift of the multiple scattering peaks at 24390 and 24420 eV compared to the reduced Pd/Al2O35. This phase appeared to be uniform along the reactor bed. To enhance the information obtainable from the XANES region, multivariate curve resolution (MCR) analysis was employed using the full series of measurements at the different temperatures to extract discrete principal components. Figure 2 shows the MCR spectra at the different reaction temperatures as well as the corresponding spatially resolved gas composition obtained from mass spectrometry.
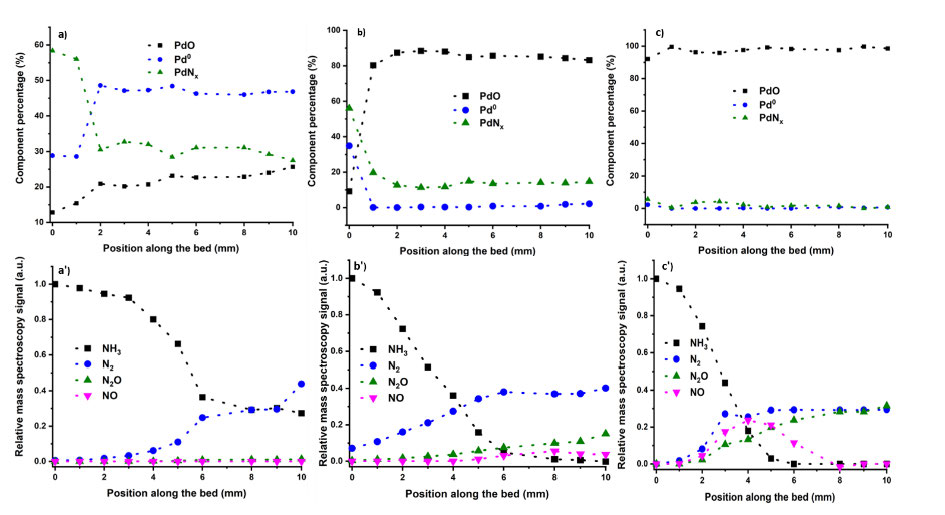
It can be seen that when the temperature increased, Pd speciation and selectivity changed significantly. At 175 °C, while the PdNx concentration decreased after 2 mm into the bed, there still was a significant XANES signature of nitride even after the light-off. These structural changes can be interpreted as a replacement of the bulk nitride structure, to one in which nitrogen is predominantly on the surface of the Pd NP, similarly to other structures such as PdCx. As temperature increased further, PdNx fraction decreased along the bed, and this was reflected in a decrease of N2 selectivity, and an increase of the NOx products. This became more apparent at 400 °C, when no PdNx could be observed in the MCR. These results highlight the need for a presence of PdNx to ensure a sufficient N2 selectivity.
Significance
This study has not only provided crucial insights into NH3-SCO over Pd/Al2O3 but it also further demonstrates the wealth of information that full spatial analysis can provide.
References
1. A. Chakrabarti et al., Catal. Today, 2017, 283, 27–53.
2. E. K. Dann et al., J. Catal., 2019, 373, 201–208.3
3. C. Stewart et al., ACS Catal., 2018, 8, 8255–8262.
4. S. J. A. Figueroa, J. Catal., 2014, 312, 69–77.
5. E. K Dann et al., Nat. Catal. 2019, 2 (2), 157–163.
Donato Decarolis1*, Peter P. Wells2*, Alexandre Goguet3*, Emma K. Gibson4
1Cardiff Catalysis Institute, School of Chemistry, Cardiff University, Cardiff CF10 3AT, UK
2UK Catalysis Hub, Research Complex at Harwell, Rutherford Appleton Lab, Harwell, Oxfordshire OX11 0FA, UK
3School of Chemistry, University of Southampton, Southampton SO17 1BJ, United Kingdom
4Diamond Light Source Ltd., Harwell Science and Innovation Campus, Chilton, Didcot OX11 0DE, United Kingdom.
5Queen’s University Belfast, School of Chemistry, David Keir Building, Stranmillis Rd, Belfast BT9 5AG, United Kingdom
6School of Chemistry, Joseph Black Building, University of Glasgow, Glasgow G12 8QQ, United Kingdom
Watch a recording of the talk below:
Biography

Dr. Donato Decarolis did his PhD at University College of London with Prof. Andrew M. Beale. His project was concerned with exploring the effect of precious metal nanoparticle size and support type on catalytic activity using in situ spectroscopy method, with a particular focus on X-ray absorption spectroscopy (XAFS). In 2017 he joined Dr. Martin Attfield group, the Centre for Nanoporous Materials, as a postdoctoral research associate. His project involved a collaboration with BP, underneath the parameters of BP-ICAM, to study the absorption of CO and N2 of Metal-organic frameworks and using X-ray absorption spectroscopy to investigate electronic or structure modification upon gas absorption. Subsequently he joined Dr. Peter P. Wells at University of Southampton in 2018, to work within the Synchrotron Techniques for African Research and Technology (START) project. Since March 2019 he joined the UK Catalysis Hub where he works as Research Associate in Synchrotron Method for Catalysis. His main role consist in developing an understanding of the structure-activity relationship of catalysts using advanced operando characterisation techniques. His particular interest lies in the application of combined spatially resolved methods in order to have improved overview of the nature of a catalyst bed and its behaviour as function of external solicitation (e.g. gas, temperature).




