Energy Theme Highlights: In-situ Probing Structure and Electronic Properties of Transition Metal Oxide Electrocatalysts
The Energy theme of the UK Catalysis has undertaken a wide range of liquid and gas phase projects related to energy conversion catalytic technologies. All the projects have involved strongly multidisciplinary teams utilising expertise from around the UK and internationally which has been facilitated by the funding mechanism from the Hub grant. In-situ Probing Structure and Electronic Properties of Transition Metal Oxide Electrocatalysts was a project which attracted attention this year.
The large-scale penetration of hydrogen as energy vector strongly depends on the performance and scalability of electrolysers and fuel cells. The efficiencies of these devices are strongly linked to the performance of the OER and ORR electrocatalysts. A number of high profile studies were published in the last couple of years proposing various descriptors for rationalising the reactivity of TMOs in this context, yet a generally accepted understanding to rationally drive catalyst optimisation has not yet emerged.
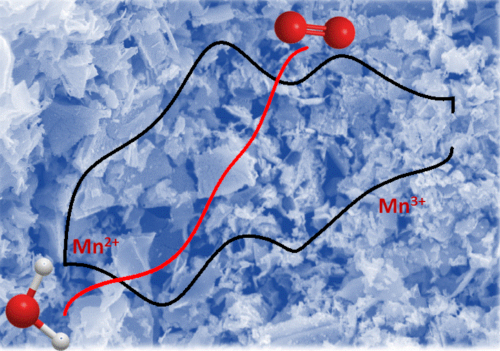
We have prepared and characterised a wide range of perovskites (ABO3) nanostructures with La, Ca, Y, Te, Ba and Sr in the A-site and Cr, Mn, Fe, Co and Ni in the B-site. The synthesis is based on a highly versatile ionic liquid method which allows the preparation of high phase purity particles in the range of 20 nm to few microns. So far, most of our studies have been focused on the oxygen reduction reaction (ORR). The compositional dependence activity of LaxCa1-xMnO3 nanoparticles towards the ORR provides an example of the type of work underpinning this project. Replacing La3+ by Ca2+ promotes a change in the initial d orbitals occupancy of the Mn site, which then affect the activity of the catalyst. The Mn oxidation state was estimated by XANES (B18 line at Diamond), while the surface composition was analysed by XPS. For the first time, we showed that the mean activity of surface Mn atoms towards the ORR increases as the d-orbital occupancy increases (decreasing oxidation state). These findings have led to a series of studies optimising the LaMnO3 synthetic conditions as well as other dopants such as Ba and Te. We have also investigated the electrocatalytic activity of Ba0.5Sr0.5CoxFe1-xO3-δ as a function of ‘x’ towards the ORR. Contrary to Mn-based catalysts, there are no changes in the oxidation state promoted by the applied electrode potential within the region relevant to ORR. In this case, the orbital occupancy of the Co active site is determined by the extent of oxygen occupancies, which is dependent of the Co/Fe ratio.
Initial studies on electrocatalysis for the oxygen evolution reaction (OER) have shown clear evidence that OH adsorption promoted by changes in redox state at Ni based-oxides is key for rationalising the high activity of these TMO catalysts. Furthermore, a series of pyrochlores with the general composition A2BB’O7 (A: Er, Ho, Dy; B: Ru, Mn) are also being investigated as dual ORR/OER electrocatalysts.
Find out more:
http://pubs.rsc.org/en/content/articlelanding/2016/cy/c6cy01105e#!divAbstract
http://pubs.acs.org/doi/abs/10.1021/acs.jpcc.6b04781?src=recsys
http://onlinelibrary.wiley.com/wol1/doi/10.1002/celc.201500440/abstract
Author:
Dr Veronica Celorrio, UK Catalysis Hub




