Design Theme highlights: Tandem Site and Size Controlled Pd Nanoparticles for the Directed Hydrogenation of Furfural
The Design theme of the UK Catalysis has undertaken a wide range of fundamental projects related to the understanding of catalytic processes, design of better catalysts and in particular developing the use of large facilities such as Diamond Light Source and ISIS neutron and muon source for catalysis research. A project of note this year has been: Tandem Site and Size Controlled Pd Nanoparticles for the Directed Hydrogenation of Furfural. The conversion of biomass to useful chemical products requires precise catalytic properties to achieve the required activity, selectivity and durability. In this study we have demonstrated, through optimized colloidal-synthesis, the tandem control of Pd size and site availability for the directed hydrogenation of the bio-derived intermediate, furfural.
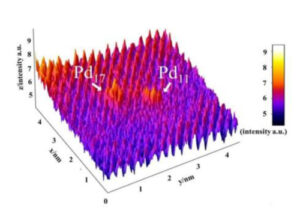
Adjusting the temperature of colloidal reduction dictates the size of Pd nanoparticles; in some instances ultra-small clusters < 20 atoms are achieved, which were identified at the Nanoscale Physics Research Laboratory at the University of Birmingham (figure 1).
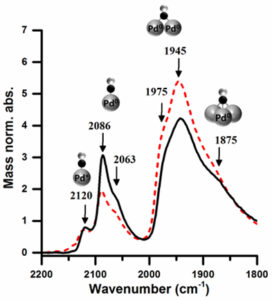
Site selective catalysis was enabled by adjusting the solvent of colloidal preparation; the Pd/TiO2 catalysts prepared using a water-ethanol solvent were found, by CO-adsorption with IR, to have a larger proportion of available corner and edge sites compared to those prepared solely in water (figure 2.) The corner and edge sites were able to direct the selectivity of the furfural hydrogenation products by adsorbing furfural perpendicular to the nanoparticle surface, resulting in greater selectivity to form furfuryl alcohol over the complete reduction product, tetrahydrofurfuryl alcohol.
The used catalyst was studied using X-ray absorption fine structure (XAFS) spectroscopy and changes from the fresh catalyst were attributed to the formation of Pd carbide, which is responsible for a reduction in catalytic activity. This assertion is supported by computational modelling studies, which show the carbidization of Pd, reduces the binding energy of furfural, leading to a reduced efficacy of furfural binding, and consequently lower catalytic activity.
This approach to nanoparticle optimization is an important strategy for producing long-lasting, high-performance catalysts for emerging sustainable technologies.
People involved – University of Milan, University of Birmingham, University College London, UK Catalysis Hub, University of Southampton, Diamond Light Source, Cardiff University
“Our approach to nanoparticle design is able to influence both the activity and selectivity of Pd NPs for selective hydrogenation reactions; this is an important step in moving towards new sustainable processes.” ~ Peter Wells
Read more:
More information – DOI: 10.1021/acscatal.6b03190
Author:
Scott Rogers, University College London




