The intrinsic ability of zeolite H-ZSM-5 to convert methanol into hydrocarbons (MTH) promises a substantial impact on the petro-chemical industry due to its ability to provide better/renewable alternatives to the conventional fossil fuel based gasoline or polymer grade olefin processes [1,2]. It has triggered extensive research to understand the underlying reaction mechanism using both experimental and computational tools [2]. Although it is widely accepted that the Brønsted acidic sites (H+–O–Al/Si) are the active sites for the MTH reaction and that the hydrocarbon pool (HCP) formed in the zeolite pores during the reaction plays a key role in the steady sate hydrocarbon production, the initial stages of the reaction including methanol adsorption, diffusion, the first C-C bond formation and the HCP composition are still a topic of debate [2-4]. In the present study, we have conducted a systematic investigation of methanol dynamics and reactivity in zeolite pores by quasi elastic neutron scattering (QENS) and operando infrared/mass spectrometry, respectively [3-6].
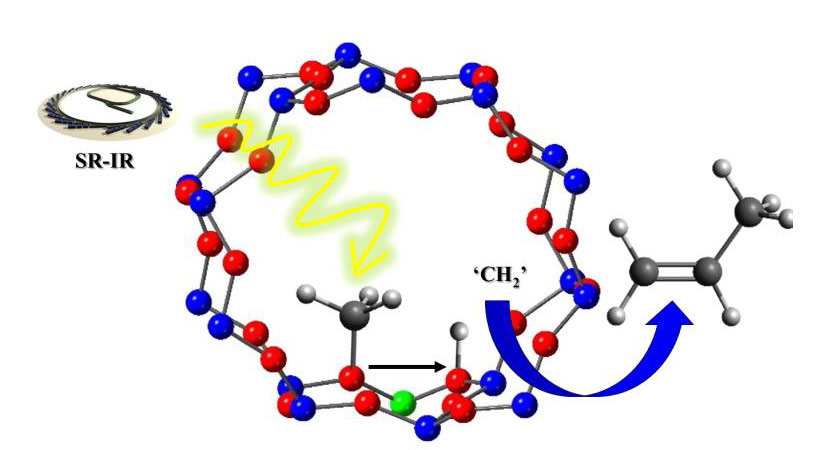
QENS shows that, at a saturation level of methanol loading in zeolite H-ZSM-5 pores, majority of methanol is immobile (in the temperature range between 298 and 373 K) and the minority is mobile which, however, presents temperature dependent dynamics [5,6]. At 298 K, the mobile methanol fraction exhibits rotational dynamics with a rotational diffusion coefficient (DR) of 4.75 x 1010s-1. Between 323 and 373 K, the mobile methanol fraction shows confined diffusion (Volino-Dianoux) within zeolite pores with a self-diffusion co-efficient (Ds) of around 8 x 10-10 m2 s-1 and jump diffusion (Chudley-Elliot) of methanol with a Ds of around 1.2 x 10-9 m2 s-1 (with a jump distance of around 2.8 Å and a residence time (τ) of around 10.8).The immobile fraction is attributed to the occurrence of both hydrogen bonded methanol with protonated geometry and methoxy species as evident from operando infrared studies [7,8]. The methoxy species dominate at 573 K and are involved directly in the first C-C bond formation via carbene like intermediates. The presentation will illustrate the elementary steps involved in the first C-C bond formation and evolution of the initial HCP composition.
Santhosh Kumar Matam1,2
1Cardiff Catalysis Institute, School of Chemistry, Cardiff University, CF10 3AT, UK
2The UK Catalysis Hub, Rutherford Appleton Laboratory, OX11 0FA, UK;
3Chemistry Department, E-mail: santhosh.matam@rc-harwell.ac.uk
References
1. Chang, C. D. Silvestri, A. J. J. Catal. 47, 249, 1977
2. van Speybroeck, V. Waroquier, M. Bell, R. G. Catlow, C. Richard. A. et al. Chem. Soc. Rev. 44, 7044, 2015
3. Minova, I. B. Matam, S. K. Greenaway, A. Catlow, C. Richard A. Frogley, M. D. Cinque, G. Wright, P. A. Howe, R. F. ACS Catal. 9, 6564, 2019
4. Minova, I. B. Matam, S. K. Greenaway, A. Catlow, C. Richard A. Frogley, M. D. Cinque, G. Wright, P. A. Howe, R. F. Phys. Chem. Chem. Phys, 22, 18849, 2020.
5. Matam, S. K. A. Catlow, C. Richard, I. Silverwood, O’Malley, A. J. Submitted to Topics in Catalysis, 2021.
6. Omojola, T. Silverwood, I. P. O’Malley, A. J. Catal. Sci. Technol. 10, 4305, 2020.
7. Matam, S. K. Howe, R. F. Thetford, A. Catlow, C. Richard A. J. Chem. Sco. Chem. Commun. 54, 12875, 2018
8. Matam, S. K. Nastase, S. A. F. Logsdail, A. J. Catlow, C. Richard A. Chem. Sci. 11, 6805, 2020

Santhosh Kumar Matam
Santhosh Kumar Matam is a research associate at Cardiff University with Prof. Sir C. Richard A. Catlow, FRS and is based at the UK Catalysis hub, STFC Rutherford Appleton Laboratories, Harwell. He earned his PhD in a German Research Foundation project from Humboldt University of Berlin, Germany. The Research Council of Norway fellowship and Swiss National Foundation grants helped him to pursue his research. Santhosh’s research activities are primarily centred on in situ/operando spectroscopy for deriving catalyst structure-activity relationships. He employs Neutrons, X-rays and Laser based techniques and closely collaborates with computational chemists with an aim to design and develop inorganic solid materials for energy and environmental applications, which include carbon neutral renewable energy and exhaust after-treatment technologies. He is also interested in operando reactors that allow real operation of chemical processes without intrinsic limitations.
View this talk below:




