Company
UK Catalysis Hub, Cardiff Catalysis Institute (Cardiff University), Mitsubishi Hitachi Power Systems Europe, Steag, Hydrogenics, i-deals, National Institute of Chemistry Slovenia, Carbon Recycling International, DIME University of Genova, University of Duisburg Essen, RWE
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement
No 637016.
Challenge
Global warming is a threat to the global environment and is a focus of a great deal of current social, political and scientific thinking due to the urgency of the problem.
A number of scenarios are in operation for the amelioration of this problem and one of them is to use renewable energy from (e.g.) wind and solar photovoltaic inputs to the grid.
However the persistent problem with this idea is the great variability of these outputs (solar photovoltaic only daytime, and not every day, wind intermittent with sometimes long windless periods).
Solution
Hence we need to store the energy during peak outputs, to be used during fallow periods. But how? A number of possibilities exist of which our work is one, that is, to store the energy at the peak in a chemical form to be used later. Ideally this could also be directly used as a fuel (e.g. to drive vehicles, or to drive turbines to regenerate electricity etc) or to recover the hydrogen in it for use in fuel cells.
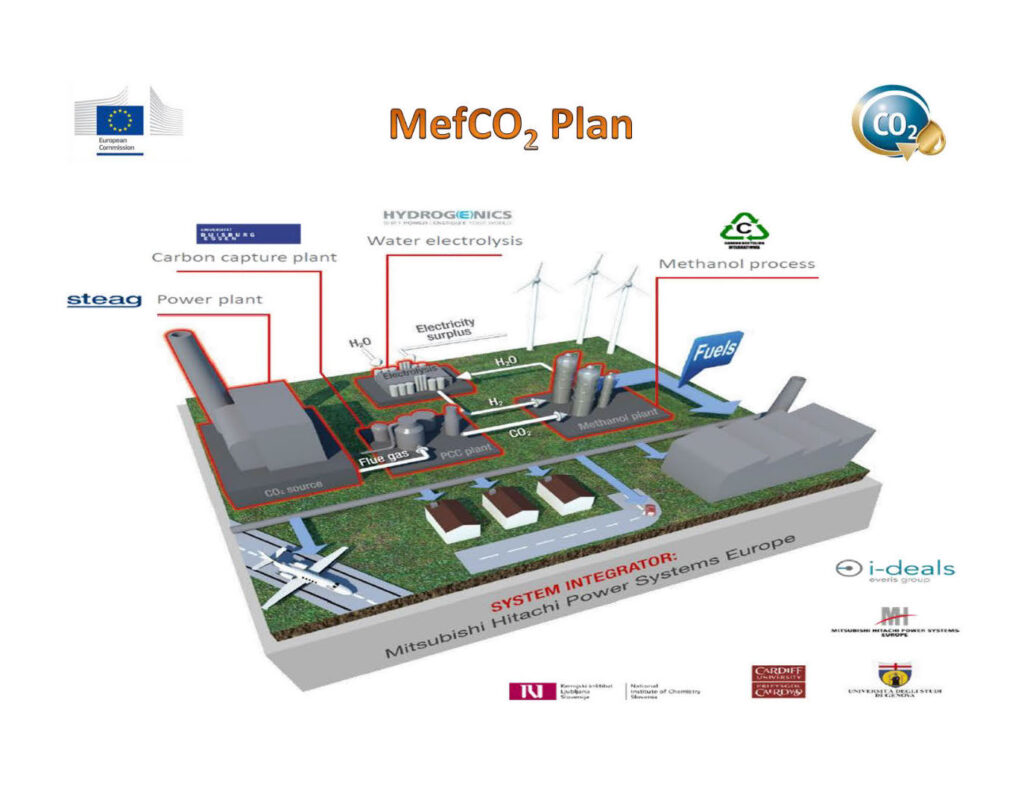
So, our work is directed at methanol synthesis, using renewables. The renewable is solar-powered electrolysis of water to produce H2 (and pure oxygen which has medical value), to react it with a hydrogen carrier (CO2 from a coal-fired power station) and to make methanol, a storable, transportable form of solar energy.
So we build a plant, for which there are several components. 1. CO2 source (a working large scale coal fired power plant in Germany) 2. CO2 clean up process 3. Hydrogen generator (electrolysis) 4. Methanol reactor/plant).
A crucial component of the latter, without which it would not work, is the methanol synthesis catalysts, and it is to this we have contributed our expertise.
Benefits
We have generated new types of catalyst (and have synthesised ~ 200 different types) and some of these are now patented for use in the plant.
The academic benefits including training for the postdoc on the work, including preparation of patent documentation. Collaboration with our European colleagues. Papers in the literature as described above, and publication of a paper related to the patent soon.
Publications
Read more about the project at http://www.mefco2.eu/
Hasliza Bahruji, Jonathan Ruiz, Esquius, Michael Bowker, Graham Hutchings, Robert D. Armstrong, Wilm Jones, Solvent Free Synthesis of PdZn/TiO2 Catalysts for the Hydrogenation of CO2 to Methanol, Top Catal 61, 144–153 (2018). https://doi.org/10.1007/s11244-018-0885-6
H. Bahruji, M. Bowker, W. Jones, J. Hayward, J. Ruiz Esquius, D. J. Morgan, G. J. Hutchings, PdZn catalysts for CO2 hydrogenation to methanol using chemical vapour impregnation (CVI), Faraday Discuss., 2017,197, 309-324, https://doi.org/10.1039/C6FD00189K
Dr. James S. Hayward, Dr. Paul J. Smith, Dr. Simon A. Kondrat, Prof. Michael Bowker, Prof. Graham J. Hutchings, The Effects of Secondary Oxides on Copper‐Based Catalysts for Green Methanol Synthesis, Chem Cat Chem., Volume9, Issue9, Special Issue: Catalysis for New Energy Technology, May 10, 2017, Pages 1655-1662, DOI: https://doi.org/10.1002/cctc.201601692




